Episode 119: AML Series, Pt 5 - AML Induction: Options for Upfront Therapy
This week, we round out our discussion regarding AML induction, this time focusing on the treatment options in the first line setting. We go through the various options, the dosing strategies, and the data behind them.
Be sure to check out parts one and two of this three-part induction discussion, as we continue to build on these concepts! Also, if you have not done so, please do check out our hemepath series to ensure you can more easily follow along with this conversation!
This episode is sponsored by our Global Research Partners! Click here to get paid to participate in market research surveys!
History of gemtuzumab ozogamicin
Mechanism of action: Gemtuzumab ozogamicin (GO) is a CD33 antibody conjugate. Immature myeloblasts express CD33 on the surface of cells, which are downregulated as the cell matures in the normal cell maturation process. In AML, this downregulation does not occur, leading to a potential target for CD33 targeted therapies. GO is a IgG4 monoclonal antibody-drug conjugate, with the antibody binding CD33, allowing the cell to internalize a highly toxic antibiotic called calicheamicin. The delivery system is specific to release only when in an acidic environment such as the myeloblast or lysosome. (Selby, 2019)
Image source: Selby, 2019. No copyright infringement intended.
Initial approval (2000-2010): GO was initially approved in 2000 for patients >60 years old or with CD33 relapsed disease. The brand name is Myelotarg.
Dosing: Initially given conditional approval at larger dose of 9 mg/m2 given on days 1 and 15 of induction based on phase 2 data in patients with relapsed disease
In 2010, FDA approval of gemtuzumab was voluntarily withdrawn after the SWOG S0106 trial failed to show a survival benefit while also noting a striking increase in early mortality (5% vs 1%) compared to placebo.
In addition, rates of veno-occlusive disease (VOD) were noted to be substantially increased (particularly in patients who would later undergo allogeneic-SCT)
Data from other trials conducted in Europe around the same timeframe also had mixed results in terms of efficacy.
Later, it was realized that differing doses of daunorubicin during induction may be a reason for these conflicting results as some studies such as SWOG S0106 used daunorubicin at a lower dose (45mg/m2) in the GO arm and a higher dose (60mg/m2) in the control arm
Reapproval (2017): In 2017, the drug was reapproved based on the trial ALFA-0701 published in Lancet 2012. In this study, patients with AML were randomized to 7+3 vs 7+3+GO, regardless of cytogenetic risk.
Dosing: Fractionated doses of 3 mg/m2 were given on days 1, 4, and 7 of induction based on prior data (in the relapsed/refractory setting) showing efficacy with reduced level of toxicity compared to the prior 9mg/m2 dosing
The fractionated dosing of GO was tolerable and led to initially improved EFS and OS at interim analysis.
The longer term follow up showed no difference in EFS or OS, so this was not adopted as standard of care.
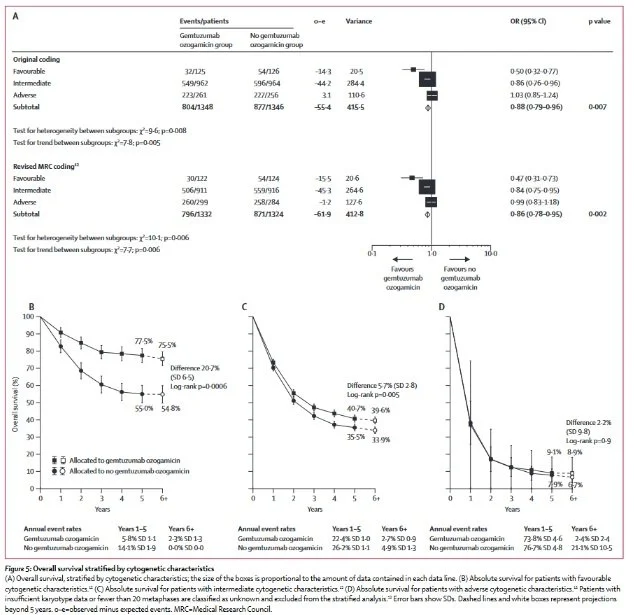
GO in favorable risk disease: In 2014, a meta-analysis looked at the pooled trial data for 7+3 and 7+3+GO evaluating the outcomes stratified by ELN risk (Hills, 2014).
The study did notably have slightly different dosing strategies, but overall limited heterogeneity.
The study showed significant benefit for favorable risk patients with 7+3+GO.
In favorable risk AML, the 5 year OS estimate was 78% vs. 55% for those with 7+3+GO compared to 7+3. Because of this meta analysis, favorable risk patients receive 7+3+GO as the standard of care at many centers.
Image source: Hills, et al., 2014. No copyright infringement intended.
MDS/AML
Refer back to Part 2 of our AML series for a detailed discussion of how to characterize MDS/AML or AML with myelodysplasia related changes
MDS/AML is marked by a characteristic cytogenetic abnormality or molecular mutation associated with an antecedent MDS in our AML patients
CPX-351
CPX-351 is a liposomal formulation of 7+3 packaged in a molar ratio of cytarabine:daunorubicin.
Encapsulation in a liposomal form ensures intracellular delivery of this specific drug ratio, enhancing uptake and cytotoxicity in leukemic cells
It is given on Day 1, 3, and 5 as opposed to continuous infusion cytarabine because we don’t have to worry about rapid cytarabine clearance in the peripheral blood
CPX-351 (Vyxeos) was studied in patients between 60-75 years old with secondary AML
An open-label multicentre phase III trial in the Lancet in 2018 studied CPX-351 (Vyxeos) vs. 7+3 chemotherapy in patients above the age of 60 with secondary AML
Secondary AML for the purposes of this study was defined as:
Patients who developed AML from an antecedent MPN such as ET or PV
Patients with therapy-related AML due to prior chemotherapy or radiation
Patients with de novo AML with MDS-related cytogenetic abnormalities (based on the 2008 WHO criteria)
Patients were randomized 1:1 to CPX-351 versus 7+3 for initial induction. The primary outcome was overall survival.
Induction:
Treatment arm: CPX-351 at 100 units/m2 (days 1, 3, 5)
Control arm: 7+3 with cytarabine at 100mg/m2/day (d1-7 continuous) and daunorubicin at 60mg/m2 (days 1, 2, 3)
Re-induction:
A re-induction regimen was permissible if patients failed to achieve CR, or CRi on first induction attempt; re-induction was required to be started by d35:
Treatment arm: CPX-351 at 100u/m2 (days 1, 3)
Control arm: 5+2 with cytarabine at 100mg/m2/day (d1-5 continuous) and daunorubicin at 60mg/m2 on (days 1, 2)
Consolidation:
Patients with CR or CRi following induction were recommended to complete up to two cycles of consolidation (5-8 weeks apart). Patients were required to have an ANC of >500 and PLT > 50 to be eligible for consolidation.
Treatment arm: CPX-351 at 65 u/m2 (days 1, 3)
Control arm: 5+2 with cytarabine at 100mg/m2/day (d1-5 continuous) and daunorubicin at 60mg/m2 (days 1, 2)
Results
CPX-351 patients had improved CR/CRi rate (48% vs. 33%) and improved OS (median 9.56 vs. 5.95 months)
Similar incidence of non-hematological adverse events between both groups
CPX-351 patients had a prolonged time to platelet and neutrophil recovery (median 35 vs 29 days)
Study Design Concerns
Study definition of secondary AML relied on cytogenetics and morphological dysplasia (as opposed to the current definition that incorporates cytogenetics and molecular mutations)
In the control arm (standard 7+3), patients who failed to achieve remission on first induction received re-induction with 5+2.
While this is technically acceptable, the more common approach (and the preferred option in ELN guidelines for patients who do not have a response on initial induction) is to utilize a regimen that differs from the original induction regimen. This typically includes switching to a regimen that contains high-dose cytarabine (like FLAG-IDA, CLAG-M, or MEC) or potentially incorporates newer agents (such as venetoclax).
In the consolidation phase, patients in the control arm received 5+2. This was a non-standard consolidation regimen, as standard of care at that time was already high-dose cytarabine.
Remember that doses of cytarabine in high-dose regimens are significantly higher (typically 2000-3000mg/m2 every 12 hours on days 1, 3, and 5) versus in continuous infusions (100mg/m2/day d1-2 in this case)
The study only included patients >60 years old - no data as of yet in younger patients
These limitations make it challenging to apply CPX-351 to patients in clinical practice
FLAG-Ida-Ven in high-risk patients
FLAG-Ida-Ven = Fludarabine, Cytarabine, G-CSF, and Idarubicin combined with Venetoclax
The different components of FLAG-Ida-Ven:
Idarubicin has 4-8 times greater potency in vitro and better CNS penetration compared to daunorubicin
G-CSF in FLAG primes leukemic cells into the S phase, where the leukemic cells are killed
Fludarabine is a purine analogue anti-metabolite
In FLAG, fludarabine is administered 4 hours before high-dose cytarabine
The role of fludarabine in this regimen is to upregulate this enzyme to convert more cytarabine into its active form
This helps prime the cell for the activity of cytarabine for more optimal killing
Cytarabine is a pro-drug converted to its active form by an enzyme intracellularly
In this regimen, cytarabine is given at much higher doses (1500-2000mg/m2/day) than in 7+3 (100mg/m2/day)
Venetoclax is a small molecule inhibitor of BCL-2 (anti-apoptotic)
Evidence for FLAG-Ida-Ven in adverse risk groups
Early phase studies show great outcomes in adverse risk groups which were historically poor
A phase Ib/II study investigated FLAG-Ida-Ven in patients with newly diagnosed (n=29) or relapsed/refractory AML
High ORR for ND-AML(97%)
A large number of patients with ND-AML (69%) proceeded to allo-SCT, with 12 month OS estimated at 94% in this group.
Preliminary data from an ongoing phase II study involving FLAG-Ida-Ven in patients newly diagnosed or relapsed/refractory AML was presented at ASCO 2024.
Similar to the prior data, there is a high MRD negative response rate in ND-AML patients (n=68, ORR 99%, 2-year OS 75%).
To date, we are still awaiting a randomized study comparing FLAG-Ida-Ven compared to 7+3
What options do we have for induction in patients with AML with a FLT3 mutation?
Remember that there are two types of FLT3 mutations: internal tandem duplication (ITD) and tyrosine kinase domain (TKD) mutations
Historically, the presence of a FLT3 mutation was considered as a poor prognostic risk factor. However, there are now approved medications that show benefit in treatment of AML with FLT3 mutations which have drastically improved outcomes and become incorporated into standard of care.
These mutations (among others with targeted options) are the reason why obtaining a mutation analysis at the time of diagnosis (and again at the time of progression/relapse) is essential
There are two agents used in induction of patients with FLT3 mutated AML currently:
Midostaurin (ITD and TKD)
Quizartinib (ITD only)
What data is there to support using midostaurin for induction in FLT3 mutated AML?
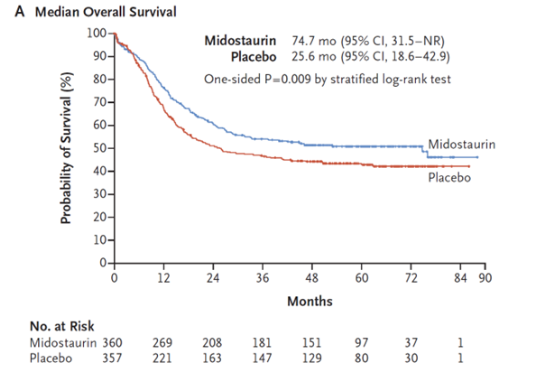
RATIFY was a randomized, placebo-controlled, double-blind phase 3 international study published in 2017 evaluating the use of midostaurin (in addition to 7+3) for induction of patients with newly diagnosed AML with a FLT3-ITD or FLT3-TKD mutation
In total, 717 patients were included in the study and baseline characteristics were balanced between both arms
Approximately 48% of patients had FLT3-ITD mutations with a low allelic ratio, 30% of patients had FLT3-ITD mutations with a high allelic ratio, and 23% of patients had FLT3-TKD mutations
Median overall survival was 74.7 months in the midostaurin group versus 25.6 months in the placebo group
Note that this was largely driven by a plateau in the KM curves experienced by both groups experienced around the 36-48 month mark; actual separation of the groups was approximately 7% in terms of 4 year OS
Image Source: Stone, et al., 2017. No copyright infringement intended.
The adverse events profiles between the midostaurin and placebo groups were largely similar, albeit with higher rates of anemia in the midostaurin group. GI side effects (nausea/vomiting, diarrhea) are common. Grade 3-5 rash was also more common with midostaurin (14.1%) vs placebo (7.6%).
Image source: Stone, et al., 2017. No copyright infringement intended.
One interesting piece of data that came from further evaluation of the RATIFY trial is that patients treated with midostaurin in the first-line setting frequently undergo clonal evolution that can result in loss or change of the actual FLT3 mutation. As a result, this highlights the importance of repeating a mutational analysis for any patients with relapsed/refractory disease at the time of progression, regardless of their initial mutation status.
Image Source: Schmalbrock, et. al, 2021. No copyright infringement intended.
What data is there to support using quizartinib for induction in FLT3 (ITD) mutated AML?
Quizartinib is a type 2 FLT3 inhibitor that is only able to bind to the inactive conformation of FLT3, which is why it is only effective for ITD mutations (and not TKD mutations, which generally favor the active conformation of the FLT3 receptor)
QuANTUM-First was a randomized, placebo-controlled, double-blind phase 3 trial published in 2023 evaluating the use of quizartinib (in addition to 7+3) as a first-line treatment in patients with FLT3-ITD mutated AML
In total, 539 patients were enrolled in the trial and baseline characteristics were relatively similar between the quizartinib and placebo arms.
Median overall survival was 31.9 months in the quizartinib group versus 15.1 months in the placebo group (with median follow-up of ~39 months in both groups)
Similar to the RATIFY data, both arms experienced a plateau in the survival curves just above (in the case of quizartinib) and below (in the case of placebo) 50%, and as a result the median OS numbers imply a more pronounced difference between the two arms than the actual separation of the curves shows.
Image source: Erba, et. al., 2023. No copyright infringement intended.
Notable adverse events with quizartinib include myelosuppression (particularly with prolonged cytopenias) and QT prolongation. In addition, rates of death within 30 days of initiating treatment were higher with quizartinib (6%) than placebo (3%), and this was largely driven by increased rates of fatal infections.
Overall, there have not been head-to-head comparisons between midostaurin and quizartinib. Both are considered appropriate options in the first-line setting for FLT3-ITD mutated AML, whereas midostaurin is the only approved option for FLT3-TKD mutated AML in the first-line setting.
This episode is sponsored by our Global Research Partners! Click here to get paid to participate in market research surveys!
The crew behind the magic:
Show outline: Vivek Patel
Production and hosts: Ronak Mistry, Vivek Patel, Dan Hausrath
Editing: Resonate Recordings
Shownotes: Sean Taasan, Karam Elsolh, Megan Connor
Social media management: Ronak Mistry
We are proud to partner with HemOnc.org!
Want to learn more about the trials that lead to the regimens discussed today? What about dosing schedules? See links in the show notes for a link to HemOnc.org
Have some extra time and want to make some extra money? Click here to get paid to participate in market research surveys!