Episode 125: AML Series, Pt 11 - Treatment of patients unfit for intensive therapy
In today’s episode, we discuss how to approach AML treatment for patients who are unfit for intensive therapy.
How do we define patients who are not fit for intensive induction?
There’s no current answer; it’s based on clinical judgement
Geriatic assessment tools do not have good prospective evidence to incorporate them into clinical practice
ECOG performance status and Karnofsky Performance status is helpful but does not capture all of patients’ comorbid conditions
This is an important area of research! We hope some of our listeners are working on this or will work on this in the future
When approaching a patients who may be unfit for intensive therapy, how do we initially manage these patients? How do we integrate cytogenetics and molecular data? How does this compare to patients who are fit for more intensive therapy?
Diagnostic testing is essentially identical for all patients with newly diagnosed AML, as discussed in Episode 116
Need to rule out and provide prophylaxis for tumor lysis syndrome and DIC
Initial stabilization is critically important with evaluation and prophylaxis for tumor lysis syndrome as well as DIC
Other important considerations:
For this patient population, starting hydroxyurea early for cytoreduction is very important given the potential risk for tumor lysis syndrome when starting venetoclax based therapy for treatment (more on this later)
We still want to rule out APL if there are any concerns by morphology or flow with prompt ATRA initiation and STAT FISH for t(15;17)
Cytogenetic testing identifying larger chromosomal rearrangements (i.e. inversions, deletions, and translocations) via FISH and karyotype and molecular analysis are still obtained
Rapid molecular testing with a panel of gene mutations is important as it results faster than comprehensive NGS and can have implications for both treatment and clinical trial options
Risk stratification differs: Based on studies looking at our modern treatment paradigm with HMA + venetoclax for these patients, the European Leukemia Network put out a consensus statement for risk stratification based on molecular data for patients receiving less intensive therapies in 2024
Adverse risk: TP53 mutation (Median OS around 5-8 months)
Intermediate risk: FLT3-ITD mutation and/or RAS (i.e., NRAS and KRAS) pathway mutation (Median OS around 12 months)
Favorable risk: Everything else (Median OS >2 years)
The study that led to this system was published essentially at the time of this recording in November 2024
It was a pooled analysis of the phase 1B trial and phase 3 RCT that led to the approval of azacitidine + venetoclax as standard of care (link here)
This new risk stratification system outperformed the ELN 2022 system and interestingly patients with MDS related gene mutations without FLT3 ITD, RAS pathway, or TP53 mutations still did well
AML risk stratification for patients unfit for intensive therapy. No copyright infringement intended.
Before we get into the mechanism of action and data behind those approvals, can we go through a historical perspective on the treatment of AML with less intensive approaches?
Check out our prior induction episode for more details but the invention of 7+3 utilizing infusional cytarabine became a standard of care treatment option after initial publication from the Roswell Park group in 1973
The problem was that patients who were above the age of 65 had a very hard time tolerating this treatment with high rates of treatment related mortality; and as we know, most patients with AML are older
The dose for infusional cytarabine was 100 mg/m2 for 7 days in a row so there was a thought to use lower doses as a subcutaneous injection for older patients
There was a small case series published in 1981 that showed cytarabine 10 mg/m2 given q12h for 7-10 doses could induce remission in patients with AML
There was a randomized phase 3 trial comparing low dose cytarabine (i.e. LoDAC) at 20 mg/m2 q12h vs. intensive induction chemotherapy in patients older than 65 published in JCO 1990
There were less CR in the LoDAC group at 32% compared to 52% in intensive induction but no difference in overall survival due to treatment related mortality for intensive induction
After that time, it was reasonable to consider low dose cytarabine as a standard care treatment option for less fit patients and was subsequently used as a control arm in future studies with CR rates generally in the single-digit percentages in subsequent studies, particularly for intermediate to poor risk cytogenetics on conventional risk stratification
In the 2000’s, there were studies that showed efficacy of hypomethylating agents in patients with MDS so the next logistical step was to trial these agents in AML
Remember that patients with AML often have epigenetic mutations resulting in downstream gene silencing through methylation
Hypomethylating agents prevent methylation and overcome these issues
There were two randomized studies comparing decitabine to standard of care (including both LoDAC and best supportive care), as well as azacitidine to standard of care
Decitabine x 5 days beat LoDAC x 10 days subcutaneous with median OS 7.7 months vs. 5.5 months with improved CR to 18% compared to 7% (link here)
There was a subsequent study looking decitabine in TP53 mutated AML and MDS which showed a 100% response rate in a single center study from U Chicago
There was another study that compared 10 days to 5 days of decitabine and it was found that 5 days was the way to go
Azacitadine x 7 days beat standard of care with LDAC, intensive chemo, or best supportive care with median OS 10 months compared to 6 months. CR rate notably 19% in the azacitidine arm (link here)
Essentially after that time, HMA monotherapy was standard of care for patients who were not fit intensive induction which sets the landscape for the current landscape with venetoclax
What is the mechanism of action of venetoclax?
Venetoclax is a BH3 mimetic that acts as a BCL2 inhibitor to induce apoptosis
Several studies had shown that AML cells have an overexpression of BCL2. BCL2 inhibits and sequesters BAX and BAK, which are pro-apoptotic.
So in the absence of BAX and BAK, there is a signal to divide
Venetoclax binds to BCL2 which frees the pro-apoptotic proteins to activate BAX and BAK leading to mitochondrial permeability. This leads to release of cytochrome c and caspase activation leading to apoptosis
Mechanism of action of venetoclax. Image source: https://thejh.org/tables/jh844t.htm. No copyright infringement intended.
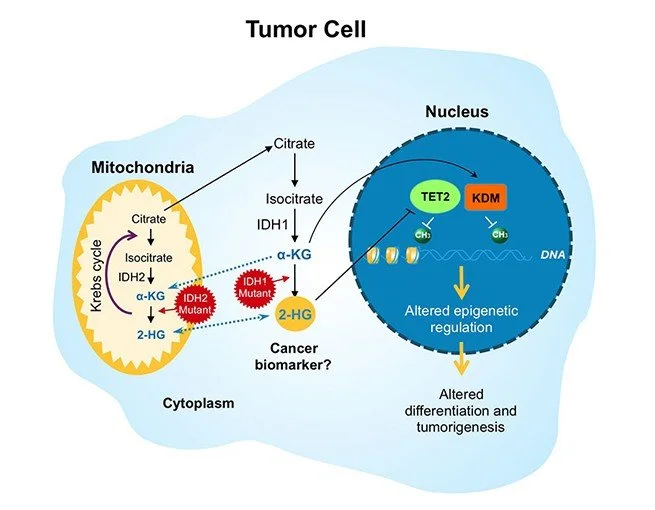
Another thing to know is that patients with IDH mutated AML are exquisitely sensitive to venetoclax. Why?
IDH1 and IDH2 are key enzymes that convert alpha-ketoglutarate in the Krebs cycle that ultimately allow for the generation of ATP in the electronic transport chain
In AML, the IDH mutations are gain of function and rapidly convert alpha ketoglutarate into another compound called 2-HG
This 2-HG prevents function of several alpha ketoglutarate enzymes that important for DNA repair and prevention of DNA hypermethylation
As a result, there is less DNA repair and a significant amount of gene silencing due to methylation
2-HG increases histone methylation in hematopoietic stem cells leading to a block in differentiation which leads to proliferation of immature myeloblasts
Last thing is that 2-HG messes with the electron transport chain in the mitochondria and inhibits cytochrome c oxidase so there is a much lower threshold to release cytochrome c and induce apoptosis
Because of this low threshold to release cytochrome c, IDH mutated cells are dependent on BCL2 overexpression for survival which makes them a prime target for venetoclax
Role of IDH in cell function. Image source: https://www.bmsscience.com/pathways/isocitrate-dehydrogenase-idh-mutations/. No copyright infringement intended.
How did we get to a doublet with venetoclax in the first line? Was it ever used as a single agent in the relapsed/refractory setting?
The first real success for venetoclax was in CLL which we discussed in a prior series
It was then tested as a single agent with escalating doses up to 800 mg daily in phase 2 study of patients with relapsed/refractory AML
The overall response rate was a dismal 19%
There was a subsequent phase 1b study that looked at HMA + venetoclax, using either decitabine or azacitidine
The study included untreated patients with AML who were not fit for intensive therapy
This was also one of the studies that was used to develop the risk stratification for less intensive induction
The composite CR rate was an astounding 61% with a median OS of 17.5 months
Based on toxicity with myelosuppression and infections, the recommended phase 2 dose was 400 mg daily
The key takeaway is that the venetoclax doublet led to much better CR rates than the 15-20% from HMA monotherapy with some patients achieving a durable remission and also we know that venetoclax single agent does not work very well
This gets us to the current standard of care for most patients with AML who are not fit for intensive induction. Can one of you go through the pivotal phase III VIALE-A study?
In this study, patients were included if they were 75 years and older:
Randomized to azacitidine x 7 days + venetoclax 400 mg daily x 28 days vs. azacitidine monotherapy
There were over 400 patients who were enrolled
The composite CR rate was 66% in the AZA + ven group and 28% in the AZA monotherapy group
In patients with IDH mutations, the CR rate was 75% which fits with the pathophysiology that we discussed
Median overall survival was 14.7 months compared to 9.6 months
The median duration of azacitidine + venetoclax was 7 cycles
There was significant myelosuppression in the aza + venetoclax arm with about 75% of patients requiring dose interruption or reduction and 25% of patients requiring discontinuation
Tumor lysis syndrome was reported in 1% of patients on the study
We discussed that myelosuppression can be an issue in patients getting HMA + ven. Where did this concept of 21 days come from and how does dose reduction work?
Check out this link for an amazing “How I Treat” article about this very topic!
One key thing to know is that most centers now start with venetoclax for 21 days and obtain a marrow on that day
If there are no evidence of blasts, then G-CSF is given to allow for improved count recovery
After that, subsequent cycles should be delayed until ANC is greater than 1000 and Plt > 50K
It is reasonable to delay up to 42 day cycles and even consider delays to 56 days after ruling out active disease and appropriate venetoclax dose reductions
If there are >5% residual blasts, then patients should immediately proceed to the next cycle of therapy without interruption
In the VIALE-A trial, 75% of all responses were seen after 1 cycle but an additional 20% of responses occurred after 2-3 cycles of therapy so it is important to not discontinue therapy early
In general, it is ok to reduce the dose and frequency of azacitidine and venetoclax for myelosuppression
The frequency of venetoclax can be adjusted down to 7 days or even 3 days per cycle
We highly encourage you to check out Table 3 in the link for a fantastic breakdown of this information; we have also included some key screenshots below.
Table 3 from “How I Treat patients with AML using azacitidine and venetoclax.” No copyright infringement intended.
Another front-line option for treatment is azacitidine + ivosidenib, an oral targeted IDH1 inhibitor. What is the data behind that?
There was a phase III RCT called AGILE that led to this approval
Patients with IDH1 mutated AML were randomized to azacitidine + ivosidenib vs. azacitidine monotherapy
One major issue was that azacitidine + venetoclax was approved shortly after this trial opened
As a result, the trial was amended to have a primary endpoint of event free survival defined by failure to achieve a complete remission at 6 months, relapse after remission, or death
This is a very strange primary endpoint but it was not powered for overall survival and there was poor recruitment in the United States given widespread approval of venetoclax
A total of 146 patients were enrolled so much smaller than the VIALE-A study and randomized 1:1 to both arms
The estimated EFS was 37% in the doublet arm vs. 12% in the monotherapy arm with a statistically significant HR of 0.33
The CR rate was 47% in the azacitidine + ivosidenib arm which is notably lower than what was seen in VIALE-A in this molecular subgroup
The median OS was 29 months in the aza + ivo arm compared to 7.9 months
What about for FLT3 ITD mutated patients? Is there a role for HMA + FLT3 inhibitor?
This was investigated in the Phase III LACEWING study that randomized patients to the FLT3 inhibitor gliterinib + azaciditine to azacitadine monotherapy
In this study 50% of patients got the FLT3 inhibitor at relapse in the control arm (which is what should happen as this is standard of care and these targeted inhibitors have efficacy)
It was published in Blood 2022 and showed no difference in median OS after about 120 patients were enrolled so the study was terminated early
What’s on the horizon?
There are triplet trials ongoing looking at HMA + venetoclax + targeted agent
One very promising regimen coming out of MD Anderson is the use of cladribine + low dose cytarabine + venetoclax alternating with azacitidine + venetoclax which has led to astounding CR rates up to 92%
This is a single center study that needs confirmation; hopefully more to come in the future!
The crew behind the magic:
Show outline: Vivek Patel
Production and hosts: Ronak Mistry, Vivek Patel, Dan Hausrath
Editing: Resonate Recordings
Shownotes: Ronak Mistry
Social media management: Ronak Mistry
We are proud to partner with HemOnc.org!
Want to learn more about the trials that lead to the regimens discussed today? What about dosing schedules? See links in the show notes for a link to HemOnc.org
Have some extra time and want to make some extra money? Click here to get paid to participate in market research surveys!