Episode 075: Intro to CAR-T, Bispecifics (BiTE), and Autologous Transplant
In this week’s episode, we pause from our discussion about DLBCL to talk about the fundamentals of CAR-T, BiTE and autologous transplants, which will lay the foundation for subsequent discussions about DLBCL. These therapies are the talk of the town and have changed/will continue to change our approach to hematologic malignancies - definitely an episode you don’t want to miss.
Understanding basic immune response to cancer
B-cells: secrete antibodies with high-affinity to specific target antigens. If tumor has an aberrant expression of non-self proteins, these antibodies could be against tumor specific antigens.
T-cells have two main sub-types and roles:
CD4+ Helper: Activate killer T-cells and Memory B-cells.
CD8+ Killer: Direct cytotoxic activity against cancer cells
T-cells do not have the same high affinity targeting such as an antibody. They are developed in the thymus and undergo “negative selection”, such that only those T-cells that have low affinity for self antigens survive. Since tumor cells express self antigens, T-cells need “co-stimulation” (from a B-cell or dendritic cell) to identify tumor cells. Once activated, T-cells have the potential to self-expand to attack tumor cells.
What are CAR T-cells?
Given above limitations of our native immune response to cancer cells, scientists thought of genetically modifying T-cell receptor to have the targeting ability of an antibody and the effector function of a CD8+ killer T cell while bypassing the normal activation steps and co-stimulation.
CAR-T stands for Chimeric antigen receptor T-cell therapy. This is a gene therapy where we modify native T-cell receptors in the lab to directly target a specific antigen and elicit an immediate cytotoxic response when bound.
Identifying a specific universal target is key. Luckily, B-cell malignancies have a universal target in CD19. This is also the reason why creating CAR T-cells for solid cancers is extremely challenging.
CAR T-cells are Chimeric because they have a genetically modified receptor that combines multiple fusion proteins to allow for direct binding to a prespecified antigen like an antibody would and kill the target with automatic self-stimulation (has an inherent co-stimulatory domain) instead of requiring extrinsic co-stimulation. They also have the ability to self-expand and are therefore “living drugs”.
Anti CD19 CAR T-cells would like all B-cells expressing CD19 receptor – normal and cancer cells. This would cause B-cell aplasia temporarily after therapy, that can be managed with IVIG to prevent infection.
Video source: Created by Agrima Mian, MD
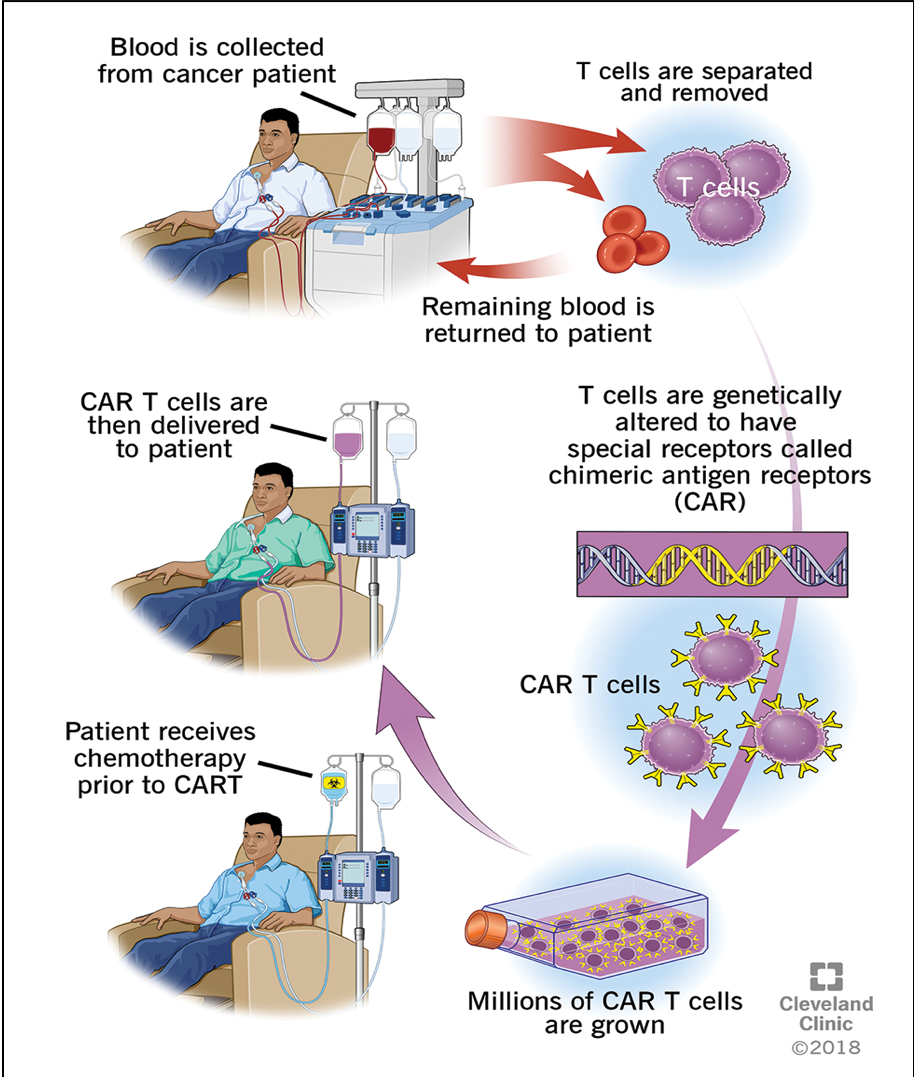
How are CAR-T cells created?
First, a letter of medical necessity is submitted by the oncologist to get insurance approval. The approval process can take a few weeks.
Once approved, the patient undergoes a process called “leukapheresis”, where patient’s cells are collected, and T-cells are separated.
These autologous T-cells are sent off to the manufacturing lab of companies that produce commercial CAR T products (eg. Kite, Novartis pharmaceuticals etc.)
Transduction of the CAR construct occurs with a retrovirus or lentivirus vector to modify the native T-cell receptors to express the target antigen receptor. These modified CAR T-cells expand over 1-2 weeks and undergo multiple quality checks.
The product is shipped back to treatment center.
Patient gets a few days of lymphodepleting chemotherapy (Fludarabine/Cyclophosphamide or Bendamustine) to get rid of native lymphocytes to not disrupt the efficacy of the product. Following this, the CAR T-cells are infused.
Overview of CAR-T cell Therapy Process
What are the commercially available CAR T-cell products for DLBCL?
There are 3 FDA approved CAR T-cell therapies for DLBCL, and all target CD19 receptor:
CD28 costimulatory domain- Rapid CAR expansion and higher risk of cytokine storm including cytokine release syndrome and neurotoxicity.
4-1BB costimulatory domain- Less rapid CAR expansion, but more persistence, and lower risk of side effects.
All CAR products have CD3z signaling domain on the T-cell receptor.
Other commercially approved CAR T products include:
Brexucabtagene autoleucel (Tecartus): Anti CD19 CAR for Mantle cell lymphoma
Idecabtagene vicleucel (Abecma): Anti BCMA CAR for Multiple myeloma
Ciltacabtagene autoleucel (Carvykti): Anti BCMA CAR for Multiple myeloma
Image source: Wells D. et al J Hematol Oncol Pharm.2022;12(1):30-42
What are the main side effects of CAR-T related cytokine storm?
Cytokine Release Syndrome (CRS): Rapid CAR T-cell expansion leads to cytokine storm, which typically sets in the first two weeks of infusion. Typically presents with SIRS like response- fever, hypotension, elevated inflammatory markers. Can progress to hypoxia and multiorgan failure in severe cases.
Immune effector cell associated neurotoxicity syndrome (ICANS): Pathogenesis is less well-understood, but likely related to cytokine storm. Presents after 10-14 days typically. Most common symptoms include tremor, aphasia, change in handwriting, and in severe cases can cause confusion, seizures, encephalopathy.
ASTCT (American Society for Transplantation and Cellular Therapy) has developed a grading system to grade the severity of CRS and ICANS.
Antidote for severe (>Grade 2) CRS: Tocilizumab (anti IL6 receptor Ab) +/- steroids
Antidote for severe (>Grade 2) ICANS: Steroids
Cytokine release syndrome (CRS) grading criteria
ICANS grading criteria
What are the current indications for use of CAR T-cell therapies in DLBCL?
Relapsed DLBCL/grade 3 FL after >2 previous lines of therapies.
Relapsed DLBCL/ grade 3 FL who relapse within 12 months of first line therapy.
Primary refractory DLBCL/grade 3 FL after first line therapy.
For patients who relapse after >12 months of first line therapy, salvage chemotherapy (RICE/R-GDP) followed by high dose chemotherapy (BEAM or BuCyVP16) and autologous stem cell transplantation (in second remission) is preferable over CAR T-cell therapy.
What is BiTE therapy, and how is it different from CAR-T?
Stands for Bispecific T-cell engager.
Essentially two antibodies fused together. Each side targets a different antigen. The BiTE acts as a “linker” or “engager”.
BiTE products for DLBCL target CD3 and CD20. This essentially means that binds to a T-cell (with CD3) to a B-cell (CD20) resulting in killing of the B-cell.
Bypasses co-stimulation, with binding of CD3 and results in death of target cell on the other side.
Off the shelf products! Skips all the manufacturing processes, cell collection etc.
Side effects can be like CAR-T cytokine storm.
Current indication for use is after progression on CAR T-cell therapy, but expect their introduction into earlier lines of therapy in the future.
Structure of BiTE. Image source: Singh, A., Dees, S. & Grewal, I.S. Overcoming the challenges associated with CD3+ T-cell redirection in cancer. Br J Cancer 124, 1037–1048 (2021). https://doi-org.ccmain.ohionet.org/10.1038/s41416-020-01225-5
Fundamentals of autologous stem cell transplants
Similar to our discussion of melphalan followed by stem cell rescue in myeloma
We give higher dose intensity and not worry about the dose limiting toxicity of marrow stem cell ablation
Difference in lymphoma is that this therapy can be curative
The key to this approach is that patients must be chemotherapy responsive before proceeding
In order to assess for chemo responsiveness, patients are first given a “salvage” regimen that must include a platinum agent (RICE and R-GDP are common)
If they achieve a PR or better after a few cycles, then we can proceed with autologous transplant
If they don’t respond to more chemotherapy, may consider CAR-T or potentially BiTE
Unlike CAR-T, the insurance approval process is extremely fast and we just need to collect their stem cells but don’t need to worry about the manufacturing time
After their salvage chemotherapy and collection, they are given a combination of chemotherapy called “BEAM” which ablates their marrow and provides the dose intensity to destroy every last remaining lymphoma cell
BCNU, etoposide, cytarabine, and melphalan
The crew behind the magic:
Show outline: Vivek Patel
Production and hosts: Ronak Mistry, Vivek Patel, Dan Hausrath
Editing: Resonate Recordings
Shownotes and images: Agrima Mian
Social media management: Ronak Mistry
We are proud to partner with HemOnc.org!
Want to learn more about the trials that lead to the regimens discussed today? What about dosing schedules? See links in the show notes for a link to HemOnc.org