Episode 107: Colorectal Cancer Series, Pt. 9 - Metastatic Colorectal Cancer (Part 1)
This week, we continue our discussion of colorectal cancer, turning our attention to metastatic disease, specifically for cancers without targetable mutations. If you have not done already, we highly recommend you check out episode 104 for our GI oncology pharmacology discussion!
This episode is sponsored by our Global Research Partners. Click here to get paid to participate in market research surveys!
What are critical pieces of data that we must pay attention to when approaching the care of a patient with metastatic colorectal cancer?
Is the disease limited or widely metastatic?
Important definitions:
Synchronous metastatic disease: found at the time of diagnosis
Metachronous metastatic disease: disease that recurs after curative treatment of an earlier state
Synchronous metastatic disease in the liver occurs in about a quarter of patients and most have localized metastatic disease to the liver
There are another 10-15% of patients who have synchronous pulmonary metastatic disease
Generally up to 5 liver or pulmonary metastases could be considered resectable and should be presented at a multidisciplinary tumor board
It is important to know that 5 year disease free survival rates with resection or radiation of metastatic disease in these highly selected cases is around 20-40%
Limited metachronous metastatic disease can also be treated in a curative manner
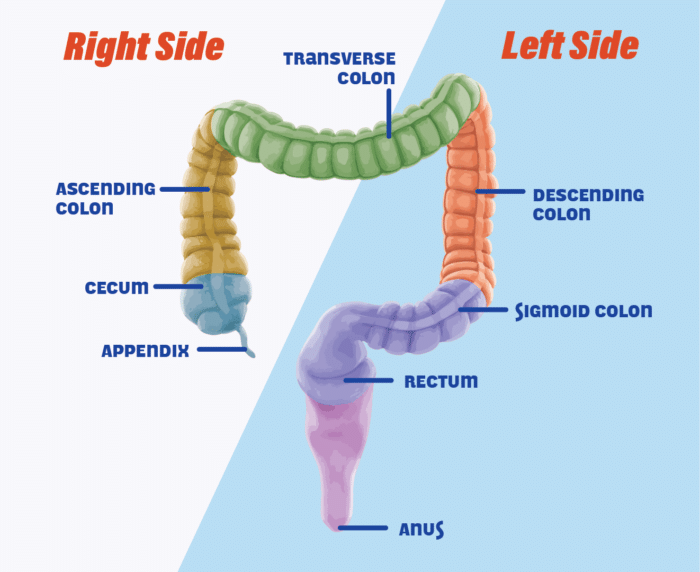
Next, we need to know if the tumor is right-sided or left-sided
Right sided is cecum to the splenic flexure
Left sided is splenic flexure onwards
Last, we need to know:
MSI status
HER2 status
BRAF status
RAS status
How did we get to our current standard of care for unresectable metastatic disease?
From the 1980-1990’s, 5-FU with leucovorin was standard of care for patients with metastatic disease with questionable survival benefit
There was a phase III randomized cooperative group trial run in 1999-2001 that compared Infusional FOLFOX4 (the 4 refers to lower 5-FU and oxaliplatin dose) vs. bolus 5-FU and irinotecan vs. irinotecan + oxaliplatin in the first line metastatic setting
FOLFOX was found to have a superior overall survival which showed us that infusional has both a better toxicity profile and efficacy compared to bolus regimens
Irinotecan and oxaliplatin combination (also known as IROX) without 5-FU was toxic with minimal efficacy
A phase III trial that really informs standard of care for most patients today run from 1997-1999 in France and Belgium
Patients with metastatic disease were randomized to FOLFIRI until progression or toxicity followed by FOLFOX or the opposite sequence
The primary endpoint was the PFS2 (total time to second progression or death)
Chemo was given every 2 weeks and the median number of cycles completed was 12 in both arms
There was no difference in response rates, PFS, or OS between the two arms
There were notable toxicity differences which are important to consider for our patients
Irinotecan arm had more alopecia and diarrhea
Oxaliplatin arm had more thrombocytopenia and neuropathy
Lastly, there were multiple efforts to understand whether CapeOx was superior to FOLFOX
CapeOx and FOLFOX were found to have similar efficacy in a meta-analysis of eight individual randomized trials
In the United States, we prefer FOLFOX given the toxicity profile and ultimate need for a port regardless with eventual use of the FOLFIRI regimen
Capecitabine with irinotecan was not tolerable given overlapping GI toxicities when studied in the metastatic setting
What is the data behind maintenance 5-FU?
Since the sequence of FOLFOX and FOLFIRI didn’t change outcomes, quality of life become the priority
In the phase III RCT called OPTIMOX1 patients were randomized to either:
FOLFOX4 q2week until progression
FOLFOX7 (higher oxaliplatin dose) q2week for 3 months → 5-FU maintenance q2week for 6 months → FOLFOX7 again for 3 months
There was no difference in PFS or OS outcomes which showed as that a “stop and go” approach is reasonable for these patients and also showed that retreatment with FOLFOX is reasonable if it was discontinued to prevent toxicity as opposed to progression
There was also less grade 3 or 4 neuropathy despite oxaliplatin dose at 85 mg/m2 in the continuous arm vs. 130 mg/m2 in the FOLFOX7 arm
There was a subsequent phase II randomized trial called OPTIMOX2 where patients were randomized to:
FOLFOX7 for 3 months → 5-FU maintenance for 6 months → retreatment with FOLFOX7 (like in OPTIMOX1)
FOLFOX7 for 3 months → reintroduction only at progression of disease
In other words 5-FU maintenance followed by retreatment vs. no maintenance followed by retreatment
The primary endpoint was duration of disease control
The arm with 5-FU maintenance had statistically significant improved duration of disease control by 3 months
The median chemotherapy free interval in the no maintenance arm was 4.6 months
There was no difference in OS but not powered for this finding
Given the improved disease control, 5-FU maintenance was solidified as a standard of care for these patients
A similar study design was used to evaluate FOLFIRI for 3 months followed by discontinuation and retreatment at progression vs. continuous FOLFIRI and showed the same results as the FOLFOX studies
There was a subsequent meta analysis looking at RCTs from 2000 to 2014 of intermittent vs. continuous oxaliplatin or irinotecan use that confirmed the non inferiority intermittent therapy followed by 5-FU maintenance
What is the role of anti-VEGF inhibitor, bevacizumab, in the management of metastatic colorectal cancer? and anti-EGFR therapies cetuximab and panitumumab?
Vascular endothelial growth factor (i.e. VEGF) was found to be an important regulator of both normal and pathologic angiogenesis
There was a humanized antibody called bevacizumab which was found to block VEGF, and the thought was that it can therefore block angiogenesis and alter the tumor vasculature to allow for optimal chemotherapy delivery
Initially there was a phase II study that combined bevacizumab with 5-FU monotherapy which showed improved response rates and survival
There was a subsequent phase III study that showed the addition of bevacizumab to bolus 5-FU and irinotecan had improved overall survival published in NEJM 2004
The efficacy with infusional regimens was still unanswered; There were numerous RCTs looking at FOLFOX + bevacizumab vs. FOLFOX + placebo
The largest study was published in JCO 2008
There were 1400 patients that were randomized to either CapeOx or FOLFOX followed by a second randomization to bevacizumab vs. placebo
The coprimary endpoints were non inferior PFS of CapeOx to FOLFOX and superior PFS of bevacizumab vs. placebo
There was no difference between CapeOx and FOLFOX as we’ve discussed
FOLFOX or CapeOx + bevacizumab had statistically significant improved PFS by 1.4 months compared to FOLFOX or CapeOx alone
There was interestingly no difference in response rates between the two groups
There was no statistically significant difference in OS
It is important to note that only 30% of patients continued bevacizumab in maintenance so some would argue that the benefit of bevacizumab comes if continued in maintenance
Nonetheless there was a very modest improvement in PFS with the combination
There was another trial done by the Dutch Colorectal Cancer Group, called CAIRO-3 trial, to investigate the role of bevacizumab in the maintenance setting
All patients got CapeOx + bevacizumab and then were randomized to capecitabine + bevacizumab maintenance vs. placebo followed by retreatment with CapeOx + bevacizumab at progression
Primary endpoint was PFS2
PFS2 was significantly improved by roughly 3 months and there was no difference in OS
This study led to more standard use of 5-FU with bevacizumab in maintenance in the United States
Another important study to highlight is a meta-analysis of six RCTs of front line bevacizumab in combination with chemotherapy for metastatic colorectal cancer
Included over 3000 patients
There was a very slight improved PFS and OS in those who received FOLFIRI up front
There was no difference in PFS or OS in those who received FOLFOX or CapeOx up front
This confirms the trial we discussed earlier that showed there really isn’t much of a benefit to add bevacizumab to FOLFOX or CapeOX
Last important thing to consider with bevacizumab is the toxicity and there was a meta-analysis that was done which showed higher treatment related mortality in patients who received bevacizumab therapy so patient selection is also important given very small benefits overall
Most common issues were death from hemorrhage, neutropenia, and GI perforation
In summary:
There is a very modest benefit on the order of 1-3 months when used in the maintenance setting with 5-FU and combined with FOLFIRI
There is very weak randomized trial and meta analytic data to support the use of FOLFOX + bevacizumab in the front line setting with a PFS benefit of likely one month
Why does the RAS-mutation status matter?
Colorectal cancer cells often have EGFR overexpression. A mutation downstream of EGFR will lead to constitutive activation
RAS wild type and BRAF wild type are predictive biomarkers for response to EGFR
There were early studies looking at the use of cetuximab combined with chemotherapy or as a single agent in the metastatic setting
A pooled analysis published in JCO 2008 showed that patients with RAS mutations did not derive benefit from EGFR inhibition while those with wild type RAS
There was a 0% response rate in those with RAS mutation vs. 40% response rate in those with RAS wild type
Image source: https://www.mdpi.com/1422-0067/18/4/752. (No copyright infringement intended)
Why does right versus left-sidedness of the tumor matter?
The CALGB/SWOG 80403 study included patients with colorectal cancer and KRAS wild type in codons 12 and 13
NOTE: This study did not include the extended KRAS, NRAS, or BRAF testing we currently do
All patients got FOLFOX or FOLFIRI at the discretion of the treating physician
They were then initially randomized 1:1:1 to add bevacizumab, cetuximab, or bevacizumab and cetuximab
The combination of chemotherapy + bevacizumab + cetuximab was too toxic and that arm was halted early
Patients were then randomized 1:1 to add bevacizumab vs. cetuximab to the chemotherapy backbone
In the overall population, there was no difference in PFS or OS
In a subgroup analysis, those with left sided tumors had a significantly improved PFS and OS with chemotherapy + cetuximab compared to chemotherapy + bevacizumab (OS 37 months vs. 32 months)
In right sided tumors, bevacizumab was superior to cetuximab (OS 24 months vs. 16 months)
PARADIGM published in JAMA 2023 to look specifically at left-sided RAS wild type patients with colorectal cancer conduced in Japan
Patients were randomized to FOLFOX + bevacizumab vs. FOLFOX + panitumumab
All patients had extended RAS testing done to confirm wild type status
There was improved OS at 38 months compared to 34 months so an absolute 4 month benefit with the addition of panitumumab compared to bevacizumab
Image source: https://fightcolorectalcancer.org/blog/sidedness-what-side-is-your-cancer-on/ (No copyright infrigement intended)
What is the data on giving these patients a chemotherapy holiday and retreatment with a prior regimen?
Individualized discussion, though many oncologists argue that 4-8 week holiday would be a reasonable time to forego 5-FU maintenance therapy
During these breaks, patients can be monitored serially with history, physical, and CEA
In the future, ctDNA testing will likely be helpful
Retreatment with a prior regimen is extremely reasonable
Remember that the OPTIMOX2 study showed that retreatment with FOLFOX after 5-FU maintenance had a response rate around 30-40%
It is unclear if we should retreat patients who progressed while on something like FOLFOX or FOLFIRI in later lines though and there is an ongoing study evaluating this question
What are treatment options after progression through 5-FU based therapy?
TAS-102 + bevacizumab was approved based on the SUNLIGHT trial published in NEJM 2023
Patients were randomized to TAS-102 with or without bevacizumab
There was improved OS with the bevacizumab combination from 7.5 months to 10.8 months
It is important to note that TAS-102 comes with some tolerability issues including cytopenias and GI side effects
Overall, the drug has very little activity with the combination showing an objective response rate of only about 7%
As a single agent, TAS-102 had a 1.6% response rate with a 2 month improvement in OS in the prior phase III RECOURSE trial that got it approved compared to placebo
This was based on the FRESCO-2 trial published in Lancet 2023
Patients were included if they had previously had all approved therapies including TAS-102
They were randomized to Fruqitinib vs. placebo
There was an improve OS of 7.4 months vs. 4.8 months favoring Fruqitinib which led to its approval
Notably, there were 30% of patients in both arms who received subsequent anti cancer therapies
We will discuss patients with MSI-high, HER2+, and BRAF mutated in our next episode!
Treatment summary for non-MSI-high patients:
FOLFOX or FOLFIRI backbone is standard of care.
We drop the chemotherapy after about 3 months and continue with 5-FU maintenance.
The addition of bevacizumab has a very modest benefit and has really shown to be most effective when added to FOLFIRI and potentially in the maintenance setting.
For left sided and both RAS and BRAF wild type tumors, we add EGFR inhibitors like cetuximab or panitumumab to our chemotherapy backbone.
If our patients progress, we just switch to the other regimen that we haven’t used before.
This episode is sponsored by our Global Research Partners. Click here to get paid to participate in market research surveys!
References:
https://ascopubs.org/doi/10.1200/JCO.2004.09.046: Phase III study establishing FOLFOX in mCRC
https://ascopubs.org/doi/10.1200/JCO.2004.09.046: Phase III study conducted in Belgium and France attempting to address sequence of FOLFOX or FOLFIRI first
https://ascopubs.org/doi/10.1200/JCO.2005.03.0106?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed : OPTIMOX1 Study evaluating impact of maintenance 5-FU
https://ascopubs.org/doi/10.1200/jco.2007.25.18_suppl.4013: OPTIMOX2 Study also evaluating role of maintenance 5-FU
https://ascopubs.org/doi/abs/10.1200/jco.2006.24.18_suppl.3582: A similar study supporting retreatement
https://www.sciencedirect.com/science/article/pii/S0923753419314140?via%3Dihub: meta-analysis of RCTs from 2000 to 2014 of intermittent vs. continuous oxaliplatin or irinotecan use that confirmed the non-inferiority intermittent therapy followed by 5-FU maintenance
https://www.nejm.org/doi/full/10.1056/NEJMoa032691: NEJM study showing benefit of adding bevacizumab
https://ascopubs.org/doi/10.1200/JCO.2007.14.9930?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed: Large meta-analysis showing adding bevacizumab to FOLFOX or CAPOX improved PFS by 1.4 months
https://www.sciencedirect.com/science/article/pii/S0140673614620043?via%3Dihub: CAIRO-3 Study establishing bevacizumab in maintenance setting
https://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-12-89: Meta-analysis of 6 RTCs evaluating adding bevacizumab in maintenance
https://jamanetwork.com/journals/jama/fullarticle/645368: Meta-analysis looking at toxicity of bevacizumab
https://ascopubs.org/doi/10.1200/JCO.2007.12.5906?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed: Pooled analysis showing RAS-mutated cancers do not respond to EGFR-directed therapy
https://jamanetwork.com/journals/jama/fullarticle/2632502: CALGB/SWOG 80403 study evaluating role of cetuximab and/or bevacizumab
https://meetings.asco.org/abstracts-presentations/123617 : CALGB/SWOG study subgroup analysis showing sidedness matters
https://jamanetwork.com/journals/jama/fullarticle/2803803: PARADIGM trial
https://www.nejm.org/doi/full/10.1056/NEJMoa2214963: SUNLIGHT study establishing role of TAS-102
https://www.thelancet.com/article/S0140-6736(23)00772-9/abstract: FRESCO-2 Study establishing role of fruquintinib
The crew behind the magic:
Show outline: Ronak Mistry
Production and hosts: Ronak Mistry, Vivek Patel, Dan Hausrath
Editing: Resonate Recordings
Shownotes: Ronak Mistry
Social media management: Ronak Mistry