Episode 088: Prostate Cancer Series, Pt. 5 - Systemic Treatment for Localized Prostate Cancer
As we continue our exploration of prostate cancer, we turn our focus to one of the earliest areas where medical oncologists are commonly involved: systemic therapy for non-metastatic prostate cancer. In this episode, we will review how to risk stratify localized prostate cancer, differences in FDA-approved prostate-specific PET tracers, how to evaluate for biochemical recurrence following surgical and radiation based treatments for localized prostate cancer, and when to consider utilizing systemic therapy in non-metastatic disease.
What are the fundamental principles that a medical oncologist should understand when thinking about localized prostate cancer?
While localized prostate cancer is typically handled by either Urology or Radiation Oncology, it is important to understand the key components that allow us to risk stratify patients
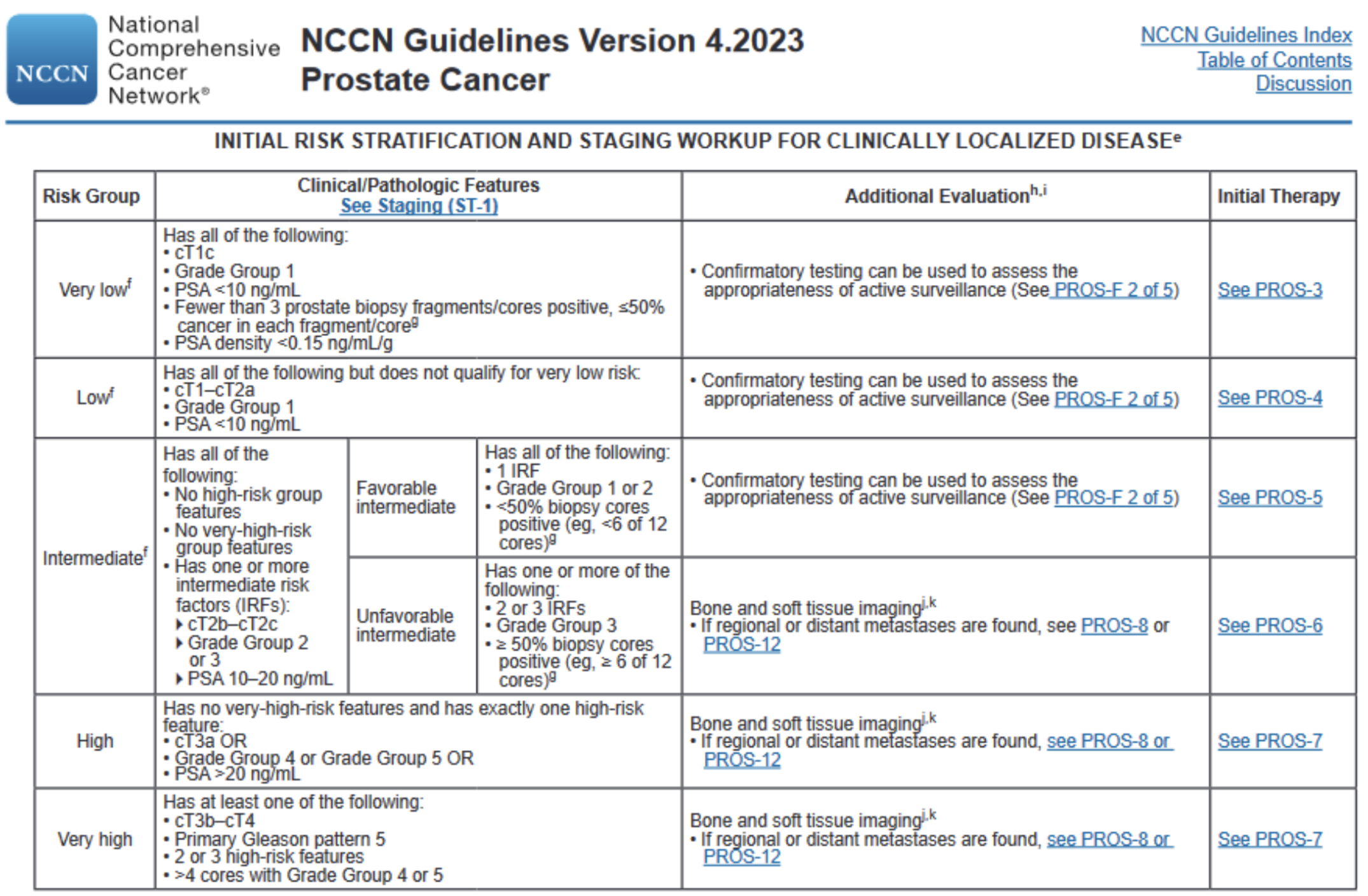
The overall risk stratification of localized prostate cancer is not based on a single factor but rather the combination of all relevant factors
There are three key questions to ask when evaluating a patient with localized prostate cancer:
What is the PSA?
What is the disease extent (ex: amount of involvement within the prostate, whether there is extraprostatic extension, whether there are regional lymph nodes present)
What is the Gleason score?
Within each of these factors, there are certain findings that are important benchmarks that contribute to an increased overall risk.
For a reminder of how to interpret these, take a look at our show notes from our introductory episode to prostate cancer (episode 084)
To determine the overall risk stratification, use the table included the NCCN guidelines (included below)
Initial definitive therapy typically involves either a surgery (radical prostatectomy +/- pelvic lymph node dissection depending on risk status) or radiation (EBRT and/or brachytherapy)
The type of initial local therapy and overall risk stratification play an important role in determining if (and what type) of adjuvant therapy is indicated
Androgen deprivation therapy (ADT) plays an increasing role in the adjuvant setting with higher risk disease
Patients who are treated with external beam radiation therapy (EBRT) and are either in the “very high” risk group or have regional node involvement should be considered for adjuvant therapy with ADT + abiraterone for 2 years as we will discuss below
Source: NCCN prostate cancer guidelines
What role does imaging play in the patient with localized prostate cancer? What are the key differences between different types of PET scans utilized in patients with prostate cancer?
For patients with a higher risk status (unfavorable intermediate, high, or very high risk), additional bone and soft tissue imaging is indicated to determine whether there is regional and/or metastatic involvement as this can significantly change management.
Bone and soft tissue imaging can be performed through a combination of different modalities (bone scintigraphy, CT, MRI, etc.), however PET is being increasingly utilized in prostate cancer
Compared to other modalities PET may provide increased ability to detect metastatic disease (particularly in patients with a low metastatic burden). This can potentially have significant treatment implications.
In one study evaluating 45 patients with high/very high risk localized prostate cancer planned for EBRT, over half of patients had either a major or minor change to their RT plan when formulated using PSMA PET rather than conventional CT. [1]
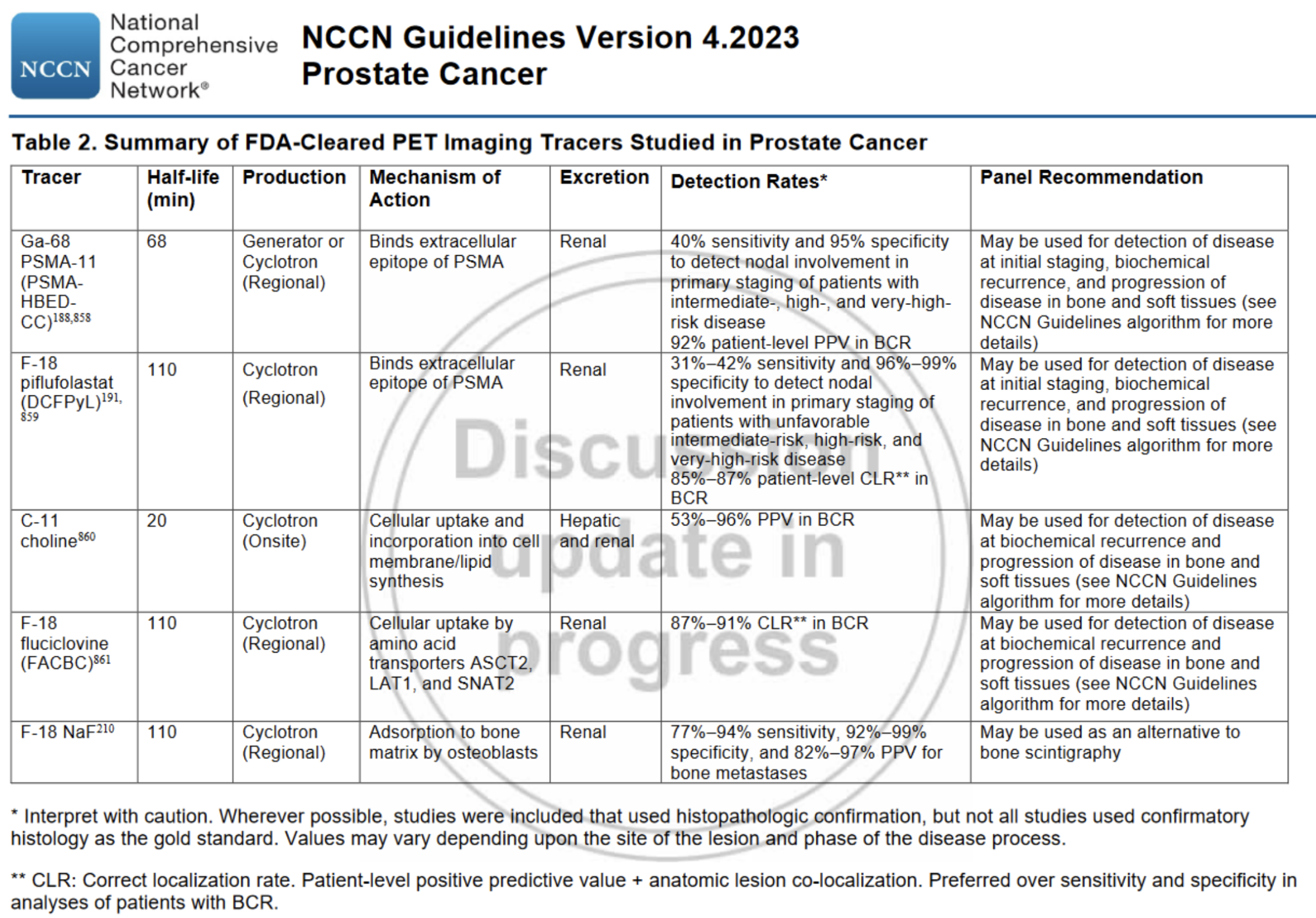
The standard FDG tracer used in many other types of cancer should not be routinely utilized when evaluating for prostate cancer. Instead, prostate-specific tracers are used.
There are currently at least six different prostate-specific tracers that are FDA approved. Broadly, these can be divided into two groups: PSMA based and non-PSMA based
PSMA based tracers are FDA approved for use in both initial staging as well as for suspected recurrence. Imaging should ideally be performed prior to starting ADT as this may decrease sensitivity.
Non-PSMA based tracers are generally only approved for suspected recurrence. F-18 NaF specifically is only used as an alternative to bone scintigraphy.
PSMA based tracers:
Ga-68 PSMA-11 (Locametz, Illuccix)
F-18 piflufolastat (Pylarify)
F-18 flotufolastat (Posluma)
Note that there is limited to no data comparing these agents directly to one another. As such, these three agents are considered generally equivalent in terms of performance and which PSMA tracer to select may be largely dictated by institutional availability and/or insurance coverage.
Non-PSMA based tracers:
C-11 choline
F-18 fluciclovine (Axumin)
F-18 NaF
For some patients with metastatic castrate resistant prostate cancer, radioligand therapy targeting PSMA-expressing cells (177Lutetium-PSMA-617, also known as Pluvicto) can be utilized. In these patients, confirmation of PSMA expression is necessary to be eligible for treatment.
The original FDA approval specifically utilized gallium-based PSMA PET scans (Locametz, Illuccix) as being appropriate for determining expression
A recent November 2023 ASCO guideline rapid recommendation update has included both F-18 piflufolastat (Pylarify) and F-18 flotufolastat (Posluma) as being appropriate for determining eligibility for 177Lu-PSMA-617 therapy. [2]
False-positives can occasionally occur, even with PSMA based tracers. As such, confirmation with biopsy or an alternative method of imaging should be considered when clinically appropriate.
In patients who undergo initial surgical management, how do we determine biochemical recurrence using the PSA?
Following initial surgical management, we do not expect a post-operative PSA rise. Patients should achieve an undetectable PSA post-operatively. As such, PSA persistence/recurrence is concerning for biochemical recurrence of prostate cancer.
PSA persistence or recurrence following a radical prostatectomy is defined as the following
Failure to achieve an undetectable PSA
Initially achieving an undetectable PSA then subsequently having a detectable PSA that increases on at least 2 measurements
Initially achieving an undetectable PSA then having a PSA >0.1 ng/mL
Patients with concern for recurrence should be considered for restaging imaging (such as with PSMA PET) to evaluate for regional/metastatic disease
Management of local/regional recurrence following radical prostatectomy typically involves salvage EBRT
For patients with local recurrence, salvage EBRT +/- ADT is the mainstay of management
For patients with regional recurrence, salvage EBRT + ADT + abiraterone for 2 years is the mainstay of management (based on data from STAMPEDE, which we will discuss shortly)
For patients with a life expectancy <5 years with local/regional recurrence, observation and deferring treatment until clinically significant progression is a reasonable approach
Source: NCCN prostate cancer guidelines
(Note: this table does not include F-18 flotufolastat, as this was approved by the FDA in May 2023 after this table was published)
In patients who undergo initial surgical management, how do we determine biochemical recurrence using the PSA?
Following initial surgical management, we do not expect a post-operative PSA rise. Patients should achieve an undetectable PSA post-operatively. As such, PSA persistence/recurrence is concerning for biochemical recurrence of prostate cancer.
PSA persistence or recurrence following a radical prostatectomy is defined as the following
Failure to achieve an undetectable PSA
Initially achieving an undetectable PSA then subsequently having a detectable PSA that increases on at least 2 measurements
Initially achieving an undetectable PSA then having a PSA >0.1 ng/mL
Patients with concern for recurrence should be considered for restaging imaging (such as with PSMA PET) to evaluate for regional/metastatic disease
Management of local/regional recurrence following radical prostatectomy typically involves salvage EBRT
For patients with local recurrence, salvage EBRT +/- ADT is the mainstay of management
For patients with regional recurrence, salvage EBRT + ADT + abiraterone for 2 years is the mainstay of management (based on data from STAMPEDE, which we will discuss shortly)
For patients with a life expectancy <5 years with local/regional recurrence, observation and deferring treatment until clinically significant progression is a reasonable approach
In patients who have previously undergone radiation therapy (either as initial definitive therapy or as salvage for recurrence following initial radical prostatectomy), how do we determine biochemical recurrence using the PSA?
Unlike following surgical intervention, patients who undergo radiation treatment often have a persistently detectable PSA which may fluctuate over time.
As such, in 2006 the RTOG and ASTRO published what are known as the Phoenix criteria to determine biochemical recurrence following radiation treatment
Following radiation therapy, the nadir of the PSA level (often ~3-6 months post-RT) is determined
An increase of >2 ng/mL above the PSA nadir (with confirmation on repeat testing) is considered biochemical recurrence in these patients
For patients who meet criteria for biochemical recurrence, restaging imaging should be performed. In addition, biopsy (of regional/metastatic sites if identified or of the prostate otherwise) should be performed to confirm recurrence.
Depending on the evaluation, patients who have local or regional recurrence may be a candidate for salvage therapy with surgery (if not performed before), brachytherapy, cryotherapy, or ADT
Even if the formal criteria are not met (PSA has increased but by < 2 ng/mL from the nadir), evaluation for recurrence should still be considered when clinically appropriate (rapidly increasing PSA, patients who are young/healthy and potentially candidates for additional salvage local therapy)
Note that increased sensitivity of PSMA PET for low-volume disease can allow for detection of recurrence that may not meet the formal Phoenix criteria yet
When should we measure testosterone levels? What is the definition of castrate-sensitive and castrate-resistant prostate cancer?
At the time of progression (biochemical or imaging), it is important to measure testosterone levels.
This is particularly important for patients have received ADT, as not all patients who receive ADT will actually have a suppressed testosterone level at the time of progression.
When patients have documented progression (biochemical or imaging), they are considered “castrate resistant” if they have a testosterone level < 50 ng/dL (< 0.5 ng/mL) at the time of progression. Otherwise, they are considered “castrate sensitive”.
How does the rate of PSA increase affect risk for future outcomes? When should we consider incorporating ADT in patients who have recurrence following initial definitive local therapy?
The rate of PSA increase (most commonly expressed as the PSA doubling time) has been associated with worse outcomes. In one study evaluating patients with biochemical recurrence following initial EBRT for localized prostate cancer, a PSA doubling time of <12 months was associated with significantly worse overall survival as well as prostate cancer specific survival. [3]
For patients who have locoregional recurrence after initial definitive local therapy and have a PSA doubling time of <12 months and a reasonable life expectancy (>5 years), we typically favor starting ADT (either by itself or in conjunction with locoregional salvage therapies, depending on the clinical scenario)
What is the difference between continuous and intermittent ADT? When should we consider using intermittent ADT?
Traditionally, ADT has been given in “continuous” fashion – with this method, patients who respond to ADT are kept on ADT indefinitely. However, there has been interest in investigating whether “intermittent” ADT (given as induction at time of recurrence, then stopped and later restarted based on PSA levels) might provide additional benefits
One theorized benefit is that intermittent ADT may reduce the risk of developing castrate-resistant disease by providing less continuous selective pressure on cells that are not dependent on PSA for growth
Another theorized benefit is that patients with intermittent ADT may have less symptoms associated with ADT, particularly those associated with cumulative ADT exposure.
Results from studies evaluating continuous vs intermittent ADT vary and likely reflect that inherent heterogeneity in the wide spectrum of prostate cancer behavior
One 2012 study in patients with non-metastatic recurrence at least >1 year after initial definitive local therapy found overall survival was non-inferior and there were some benefits in terms of quality of life symptoms [4]. One caveat was that patients with a Gleason sum >7 had a median OS of 8 years vs 6.8 years with continuous vs intermittent ADT, however the study was not powered to evaluate this.
Another 2016 study in patients with non-metastatic recurrence found no difference in clinical outcomes (PFS, OS, quality of life, adverse events) with continuous vs intermittent ADT. [5]
One study evaluating intermittent ADT in the metastatic setting found that intermittent ADT did not meet the pre-specified non-inferiority criteria for overall survival [6]
Two meta analyses from 2013 and 2015 found no significant difference in terms of overall mortality or disease progression [7,8]
While data regarding impact on survival is somewhat mixed, there does appear to generally be improved quality of life with intermittent ADT compared to continuous ADT. As a result, the NCCN recommends considering intermittent ADT in the non-metastatic recurrent setting.
What should we do when a patient has non-metastatic recurrence while already on ADT?
As noted above, it is important to check testosterone levels at the time of progression. Even if a patient is receiving ADT, some patients do not achieve chemically castrate hormone levels.
If a patient has non-castrate (above 50 ng/dL) levels of testosterone at time of recurrence despite being on ADT, additional hormone suppression (with alternate medical castration methods or surgical castration) can be considered
If a patient is confirmed to have castrate (< 50 ng/dL) levels of testosterone at the time of non-metastatic recurrence, they are considered to have non-metastatic (M0) castrate resistant prostate cancer (CRPC)
In patients with non-metastatic CRPC, ADT should still be continued. Following recurrence, the PSA doubling time should be calculated and used to help determine whether additional therapy is indicated.
A 2005 study intending to evaluate the role of bisphosphonates in non-metastatic CRPC was terminated early due to a low event rate, however data from the placebo group provided useful information regarding the natural history of patients with non-metastatic CRPC. [9]
Based on this data, a PSA doubling time of >10 months was associated with a relatively indolent course compared to those with a PSA doubling time <10 months.
As a result, patients with non-metastatic CRPC who have a PSA doubling time >10 months are typically monitored
For patients with non-metastatic CRPC and have more rapid PSA changes (PSA doubling time <10 months), there are 3 second-generation androgen receptor signaling inhibitors (ARSi) that have been approved by the FDA: apalutamide, darolutamide, and enzalutamide
All three medications received approval following phase III RCTs comparing ADT + ARSi vs ADT alone in non-metastatic CRPC with PSA doubling time <10 months (SPARTAN, ARAMIS, and PROSPER, respectively) [10,11,12]
Initial data in all three trials demonstrated improvements in the primary endpoint of metastasis-free survival by ~20 months. Symptomatic disease progression was also improved, albeit to a somewhat lesser extent (~10% absolute improvement).
Updated analyses for all three trials demonstrated overall survival benefits
One major caveat is that these trials were not designed to study when an ARSi should be utilized. Rates of using an approved therapy for CRPC upon progression varied in the placebo group across trials, from ~24% (darolutamide study) to 78% (apalutamide study).
The question of whether starting treatment sooner changes the overall trajectory of disease (as opposed to waiting until symptomatic progression occurs and starting at that time) remains somewhat unclear for this reason
Overall, it is important to individualize the decision of whether to start an ARSi vs continue to monitor for each patient. In particular, factors such as quality of life, adverse events, cost, and patient preferences should all be relevant considerations.
What is the STAMPEDE trial? Why do I keep hearing it referenced in multiple different stages/treatments for prostate cancer (and why is it so confusing to know what publication my attending is referring to when they say STAMPEDE)?
STAMPEDE is a landmark multi-arm, multi-stage trial platform. In this design, there is one “standard of care” arm that has continuous recruitment. Patients can also be randomized to one of multiple intervention arms.
The benefit of this design is that it allows each intervention arm to be compared to the control without requiring an entirely new trial infrastructure to be created
For this reason, you will see multiple publications under the “STAMPEDE” umbrella that evaluate different clinical scenarios/interventions. This can be very confusing at first if you are not familiar with the platform design.
The comparison in the arms we will focus on in this episode are centered on the addition of abiraterone to ADT + EBRT in the highest risk locoregional prostate cancer patients. [13]
In addition to the benefit of abiraterone to ADT + EBRT in high risk locoregional disease, there are a few other publications from STAMPEDE that you may hear about. This includes:
The benefit of docetaxel to ADT over ADT alone in metastatic patients (a separate publication from 2016 which eventually led to docetaxel becoming standard of care) [14]
The benefit of abiraterone in metastatic disease (included in the original 2017 publication and later separated out in future publications from non-metastatic patients) [15]
The benefit of EBRT in low-volume (<4 bony metastases and no visceral metastases) metastatic disease in addition to ADT + abiraterone (published in 2018) [16]
What should we do in patients who are in the highest risk categories for locoregional disease based on the STAMPEDE trial?
Based on the STAMPEDE trial, there is data showing benefit for the addition of 2 years of abiraterone to EBRT + ADT in the highest risk patients with locoregional disease. One key item to consider is that the definition of “high-risk” varies across trials and is not always identical to NCCN guidelines. For the purposes of the STAMPEDE trial, “high-risk” was defined as any of the three following categories:
Any regional node involvement (N1 disease) whether in newly diagnosed or relapsed setting
Meeting at least 2 of 3 criteria: T3 or T4 staging, Gleason sum score 8-10, and PSA ≥ 40ng/mL
Relapse with high-risk features: ≤12 months of total ADT with an interval of ≥12 months without treatment and PSA concentration ≥4 ng/mL with a doubling time of <6 months, or a PSA concentration ≥20 ng/mL
Note that all patients with localized disease who met STAMPEDE high-risk criteria would be considered “very high” risk by NCCN guidelines, but not all patients who are “very high” risk by NCCN will automatically meet the STAMPEDE criteria
There were two trials within the STAMPEDE platform that evaluated the role of abiraterone in the high-risk locoregional setting. In both of these cases, “SOC” was considered ADT + EBRT.
The first trial evaluated patients from 2011-2014 and compared SOC + abiraterone to SOC alone.
The second trial evaluated patients from 2014-2016 and compared SOC + abiraterone + enzalutamide to SOC alone
Data from the high-risk locoregional patients in these two trials were combined as part of a meta-analysis in 2021. [13] Key findings in high-risk locoregional patients including the following:
Improved metastasis-free survival (80% vs 70% at 6 years) with SOC + abiraterone vs SOC alone (primary endpoint)
Improved overall survival (HR 0.60) and prostate cancer specific survival (HR 0.49)
Improved progression free survival (HR 0.44)
No additional benefit was seen with adding enzalutamide on top of SOC + abiraterone, but increased toxicity was noted
These data have led EBRT + ADT + abiraterone to become standard of care in patients who are considered high-risk based off STAMPEDE criteria (all patients with regional disease, the majority but not all with “very-high risk” stratification based off NCCN criteria)
While this data is overall strong, some key limitations when interpreting this meta-analysis include the following:
Subsequent therapy following progression in the control arm is not fully clear (as such, whether the benefit is receiving abiraterone is larger upfront as opposed to waiting until further progression)
Whether upfront abiraterone improved quality of life / reduced symptom burden compared to waiting for further progression before starting abiraterone is unclear
References:
[1] Wu SY, et al. Impact of Staging 68Ga-PSMA-11 PET Scans on Radiation Treatment Plansin Patients With Prostate Cancer. Urology. 2019 Mar;125:154-162. doi: 10.1016/j.urology.2018.09.038.
[2] Garje R, et al. Systemic Therapy Update on 177Lutetium-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer: ASCO Guideline Rapid Recommendation Update. J Clin Oncol. 2023 Nov 6:JCO2302128. doi: 10.1200/JCO.23.02128.
[3] D'Amico AV, et al. Determinants of prostate cancer-specific survival after radiation therapy for patients with clinically localized prostate cancer. J Clin Oncol. 2002 Dec 1;20(23):4567-73. doi: 10.1200/JCO.2002.03.061.
[4] Crook JM, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012 Sep 6;367(10):895-903. doi: 10.1056/NEJMoa1201546. Erratum in: N Engl J Med. 2012 Dec 6;367(23):2262.
[5] Schulman C, et al. Intermittent Versus Continuous Androgen Deprivation Therapy in Patients with Relapsing or Locally Advanced Prostate Cancer: A Phase 3b Randomised Study (ICELAND). Eur Urol. 2016 Apr;69(4):720-727. doi: 10.1016/j.eururo.2015.10.007.
[6] Hussain M, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013 Apr 4;368(14):1314-25. doi: 10.1056/NEJMoa1212299.
[7] Niraula S, et al. Treatment of prostate cancer with intermittent versus continuous androgen deprivation: a systematic review of randomized trials. J Clin Oncol. 2013 Jun 1;31(16):2029-36. doi: 10.1200/JCO.2012.46.5492.
[8] Dong Z, et al. Intermittent hormone therapy versus continuous hormone therapy for locally advanced prostate cancer: a meta-analysis. Aging Male. 2015;18(4):233-7. doi: 10.3109/13685538.2015.1065245.
[9] Smith MR, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005 May 1;23(13):2918-25. doi: 10.1200/JCO.2005.01.529
[10] Smith MR, et al; SPARTAN Investigators. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018 Apr 12;378(15):1408-1418. doi: 10.1056/NEJMoa1715546
[11] Fizazi K, et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2019 Mar 28;380(13):1235-1246. doi: 10.1056/NEJMoa1815671.
[12] Hussain M, et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2018 Jun 28;378(26):2465-2474. doi: 10.1056/NEJMoa1800536.
[13] Attard G, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022 Jan 29;399(10323):447-460. doi: 10.1016/S0140-6736(21)02437-5.
[14] James ND, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016 Mar 19;387(10024):1163-77. doi: 10.1016/S0140-6736(15)01037-5.
[15] James ND, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017 Jul 27;377(4):338-351. doi: 10.1056/NEJMoa1702900.
[16] Parker CC, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018 Dec 1;392(10162):2353-2366. doi: 10.1016/S0140-6736(18)32486-3.
The crew behind the magic:
Show outline: Vivek Patel
Production and hosts: Ronak Mistry, Vivek Patel, Dan Hausrath
Editing: Resonate Recordings
Shownotes: Sean Taasan
Social media management: Ronak Mistry
We are proud to partner with HemOnc.org!
Want to learn more about the trials that lead to the regimens discussed today? What about dosing schedules? See links in the show notes for a link to HemOnc.org