Episode 127: Testicular Cancer Series, Pt 1 - Introduction
It’s time for another new series, this time focusing on Testicular Cancer. In this first episode, we lay the foundation for our future discussions and discuss the basics of Testicular Cancer.
Testicular Cancer is a type of germ cell tumor, an uncommon malignancy, accounting for less than 1% of all male tumors however due to the advancement in multimodal therapy, it is among the most curable solid tumors. [1,2]
What are germ cell tumors (GCTs)?
Germ cell tumors are a group of neoplasms that originate from primordial germ cells (precursors to sperm and eggs). Due to the migration of germ cells during embryonal development, GCTs can occur in both gonadal (testes and ovaries) and extragonadal sites (mediastinum, retroperitoneum, and CNS). [3,4]
GTCs are classified based on histological characteristics into two main categories: seminomas (or dysgerminomas in females) and non-seminomas.
Mixed germ cell tumors contain more than one histologic subtype. They can include various combinations of seminoma, embryonal carcinoma, yolk sac tumor, choriocarcinoma, and teratoma. They are classified as a nonseminoma due to the more aggressive nature of nonseminomatous components. [5]
Let’s discuss testicular germ cell tumors (TGCTs).
Testicular germ cell tumors are the most common type of malignancy in young men between the age 15-40 years. Pure seminomas account for up to 50% of testicular germ cell tumors with peak incidence between ages 25-29, and non-seminomas between 35-39. [6]
What are some risk factors for TGCTs?
The established risk factors for TGCTs include cryptorchidism, contralateral testicular GCT, and familial history of testicular cancer.
Obesity, smoking and alcohol use are not considered risk factors. [7]
Tumor Markers
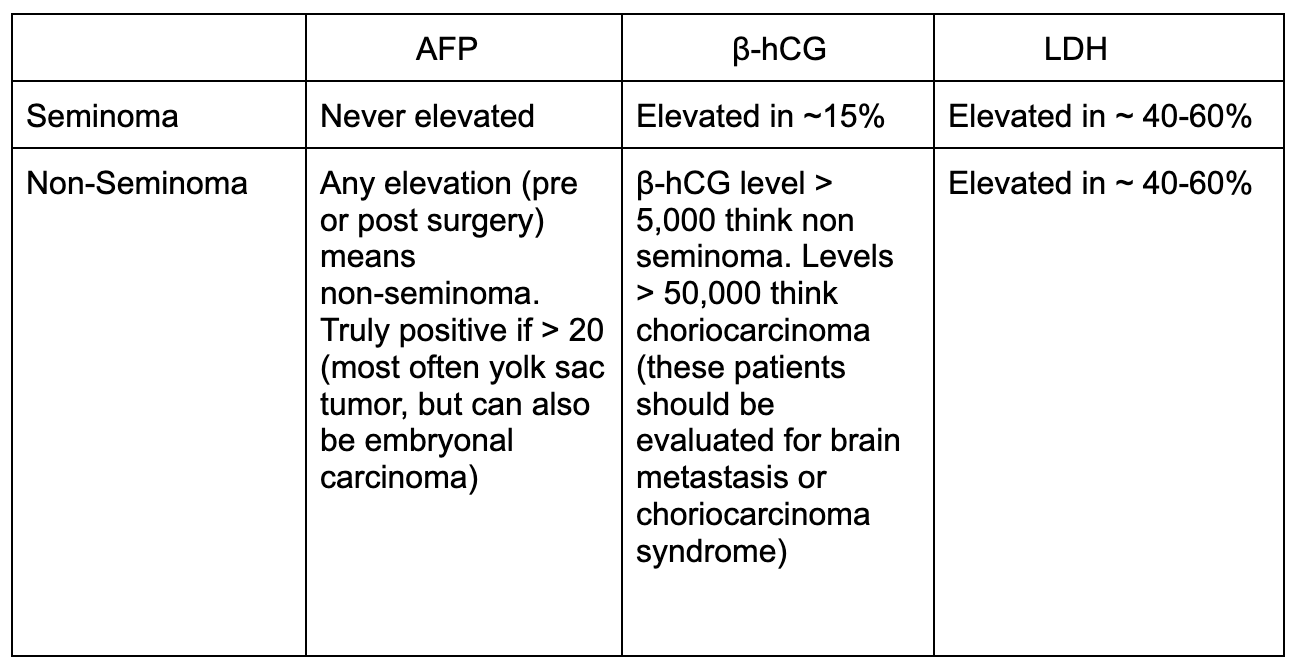
In TGCTs, 3 tumor markers are commonly tested: α-fetoprotein (AFP), human chorionic gonadotropin (β-hCG), and lactate dehydrogenase (LDH). [8]
Characteristics of Seminoma versus Non-Seminoma [9,10]
Image Source: Katabathina 2021. [11] No copyright infringement intended.
Testicular Anatomy and Staging Considerations
The testes and epididymis receive innervation from the testicular plexus and are supplied by testicular arteries (which arise directly from the abdominal aorta) along with branches of the cremasteric artery.
The venous drainage is particularly through the pampiniform plexus in the scrotum.
In terms of the lymphatics, the testes are originally a retroperitoneal organ therefore they drain into the para-aortic nodes and lumbar nodes and skip the pelvic lymph node system. [11]
Due to the lymphatic structure, it is crucial to stage with CTAP to evaluate for retroperitoneal lymph node involvement.
Additionally, CT Chest/CXR can be included to evaluate for dissemination to the mediastinum or lungs.
Image Source: Navaneethabalakrishnan 2020. [12] No copyright infringement intended.
Staging for TGCTs
Before initiating treatment, assessment of the disease extent is crucial.
Stage 1: localized disease
Stage 1S: localized disease, however may have abnormal serum tumor markers
Stage 2: Retroperitoneal lymph node involvement only
Stage 3: Involvement of any other areas, including pelvic node involvement, pulmonary, liver, and brain metastasis
There is no stage 4 Testicular cancer.
Surgical Approach for Testicular Cancer [13]
An inguinal orchiectomy is performed and preferred approach for testicular cancer for several reasons:
Diagnostic and Therapeutic approach
Prevention of tumor seeding, inguinal approach avoids the scrotum thereby reducing the risk of the tumor seeding/change of local recurrence
Staging and prognosis: accurate staging and assessment of serum tumor markers before and after orchiectomy to guide future treatment
A transscrotal approach is not recommended as it is associated with 2.5x higher rate of local recurrence and may require additional surgical interventions for scar excisions
Tumor Markers after Orchiectomy
The half life of beta HCG is approximately 1.5-3 days and the half life of AFP is 5-7 days [8]
Risk stratification relies on tumor markers at the first day of chemotherapy so it's important to wait for the nadir which can take 5-8 weeks post orchiectomy
Be patient, but begin treatment if the tumor markers start to rise at any point as nadir has been established. Rising tumor markers can indicate disseminated disease even without radiographic evidence.
Risk Stratification
ASCO provides detailed guidelines on risk stratification based on serum tumor markers7
Risk stratified as good, intermediate, or poor risk
Seminoma can only be stratified as favorable or intermediate risk as there is no tumor marker elevation
Good Risk: Isolated disease, retroperitoneal lymph nodes and/or lung involvement
Intermediate Risk: Any metastatic disease outside of the retroperitoneal lymph nodes or lungs
Non-Seminoma uses the nadir of AFP, β-hCG, and LDH to risk stratify. Can use mnemonic 1, 5, and 1.5 as intermediate and poor risk are treated similarly
Good Risk: AFP < 1,000, β-hCG < 5,000, or LDH < 1.5x upper limit of normal (ULN)
Intermediate Risk: AFP > 1,000, β-hCG > 5,000, or LDH > 1.5x ULN
Poor Risk: AFP > 10,000, β-hCG > 50,000, or LDH > 10x ULN
Disseminated disease with intermediate or poor risk cannot be treated with just etoposide and cisplatin (EP) chemotherapy regimen, must include bleomycin (BEP) or ifosfamide (VIP).
Don’t worry, we will cover this more in this series! Stay tuned.
When to evaluate for Brain Metastasis?
Patient is having concerning neurological symptoms
AFP greater than 10,000, β-hCG > 5,000
Radiographic imaging reveals extensive pulmonary involvement, non pulmonary visceral metastasis, and/or extensive involvement with predominant choriocarcinoma pathology
What from the pathology report can influence management?
The presence of lymphovascular invasion, invasion of the scrotum and predominant embryonal histology are risk factors for relapse
Teratomas are not responsive to chemotherapy or radiation thus require surgical resection. Therefore, patients with non seminoma who have any residual tumor must undergo surgical resection of the residual masses.
Growing teratoma syndrome is observed in patients with rapid progression of tumor during chemotherapy and should undergo surgical resection as soon as possible. [14]
References
1. Hwang MJ, Hamza A, Zhang M, et al. Somatic-Type Malignancies in Testicular Germ Cell Tumors: A Clinicopathologic Study of 63 Cases. Am J Surg Pathol. 2022;46(1):11-17.
2. Znaor A, Lortet-Tieulent J, Jemal A, Bray F. International variations and trends in testicular cancer incidence and mortality. Eur Urol. 2014;65(6):1095-1106. doi:10.1016/j.eururo.2013.11.004
3. El-Zaatari ZM, Ro JY. Mediastinal Germ Cell Tumors: A Review and Update on Pathologic, Clinical, and Molecular Features. Adv Anat Pathol. 2021;28(5):335-350.
4. Oosterhuis JW, Looijenga LHJ. Human germ cell tumours from a developmental perspective. Nat Rev Cancer. 2019;19(9):522-537.
5. Gilligan T, Lin DW, Aggarwal R, et al. Testicular Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(12):1529-1554.
6. McGlynn KA, Devesa SS, Sigurdson AJ, Brown LM, Tsao L, Tarone RE. Trends in the incidence of testicular germ cell tumors in the United States. Cancer. 2003;97(1):63-70.
7. Dieckmann KP, Pichlmeier U. Clinical epidemiology of testicular germ cell tumors. World J Urol. 2004;22(1):2-14.
8. Gilligan TD, Seidenfeld J, Basch EM, et al. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28(20):3388-3404.
9. Motzer RJ, Jonasch E, Agarwal N, et al. Testicular Cancer, Version 2.2015. J Natl Compr Cancer Netw JNCCN. 2015;13(6):772-799.
10. Chieffi P, Chieffi S. Molecular biomarkers as potential targets for therapeutic strategies in human testicular germ cell tumors: an overview. J Cell Physiol. 2013;228(8):1641-1646.
11. Katabathina VS, Vargas-Zapata D, Monge RA, et al. Testicular Germ Cell Tumors: Classification, Pathologic Features, Imaging Findings, and Management. RadioGraphics. 2021;41(6):1698-1716.
12. Navaneethabalakrishnan S, Goodlett BL, Lopez AH, Rutkowski JM, Mitchell BM. Hypertension and reproductive dysfunction: a possible role of inflammation and inflammation-associated lymphangiogenesis in gonads. Clin Sci (Lond). 2020;134(24):3237-3257.
13. Koschel SG, Wong LM. Radical inguinal orchidectomy: the gold standard for initial management of testicular cancer. Transl Androl Urol. 2020;9(6):3094-3102.
14. Vaughn DJ, Flaherty K, Lal P, et al. Treatment of growing teratoma syndrome. N Engl J Med. 2009;360(4):423-424.
The crew behind the magic:
Show outline: Vivek Patel
Production and hosts: Ronak Mistry, Vivek Patel, Dan Hausrath
Editing: Resonate Recordings
Shownotes: Suju Ojha, Matt Barke, Neil Biswas
Social media management: Ronak Mistry
We are proud to partner with HemOnc.org!
Want to learn more about the trials that lead to the regimens discussed today? What about dosing schedules? See links in the show notes for a link to HemOnc.org
Have some extra time and want to make some extra money? Click here to get paid to participate in market research surveys!