Episode 096: Treatment of CLL
We continue on our journey through chronic lymphocytic lymphoma (CLL), this time focusing our attention to the treatment of CLL.
Recap of last episode:
Most do not need bone marrow biopsy. They can use peripheral blood using flow cytometry and FISH to identify CD5+ CD200+ cells.
What are the indications for treatment?
Anemia and/or thrombocytopenia related to CLL infiltration of the marrow
Recurrent autoimmune cytopenias related to CLL after initial treatment with steroids +/- rituximab
Presence of B symptoms
Lymphocyte doubling time of less than 6 months (weakest reason to start treatment)
If a patient is asymptomatic with WBC < 100K, the patient’s are okay to observe.
Table source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10443285/
Does early treatment impact the trajectory of disease?
The CLL12 study published in 2022 evaluated ibrutinib vs placebo in treatment-naive patients with early-stage CLL
Patients had early-stage CLL (defined as Binet Stage A) and were asymptomatic, however patients with intermediate/high/very-high risk of progression (based on the German CLL study group risk score) were randomized 1:1 to ibrutinib/placebo
While ibrutinib did improve the primary endpoint of 3-year event free survival (85% vs 60%), there was no change in overall survival.
Overall, these results are interpreted to show that a watch-and-wait strategy is still a reasonable and preferred approach in asymptomatic patients (including those at higher risk for eventual progression).
Historically, what were the typical chemoimmunotherapy regimens utilized for CLL prior to the introduction of modern-day (BTKi and BCL2i-based) regimens?
Until the last ~5 years, chemoimmunotherapy was the primary method of treating CLL. The standard chemoimmunotherapy regimen utilized was FCR (fludarabine/cyclophoshamide/rituximab)
The CLL10 trial was a non-inferiority trial evaluating BR vs FCR, and BR did not meet its prespecified non-inferiority threshold compared to FCR
Some patients with low-risk CLL were able to effectively be cured following first-line treatment with FCR
While other regimens such as BR (bendamustine/rituximab) and chlorambucil were also available, FCR was the preferred regimen for these reasons for those who were fit enough to tolerate the additional toxicity
Why do we generally use obinutuzumab over rituximab for anti-CD20 therapy in CLL?
While rituximab was the original anti-CD20 agent used for CLL, trial data has demonstrated additional benefit with the use of a second-generation anti-CD20 agent (obinutuzumab)
Obinutuzumab is a type II anti-CD20 antibody which has increased antibody-dependent cellular cytotoxicity compared to rituximab.
The CLL11 trial compared obinutuzumab/chlorambucil versus rituximab/chlorambucil and found a PFS benefit of 26 vs 11 months on the initial analysis
Infusion reactions and neutropenia (but not infections) were more common with obintuzumab/chlorambucil compared to rituximab/chlorambucil, and overall both treatments were relatively well tolerated
A final updated analysis was presented in 2018 with a median follow-up time of ~59 months. In this updated analysis, the previously noted PFS benefit was confirmed (updated to 29 vs 16 months). Prolonged time to next treatment (56 vs 35 months) was also noted. In addition, an OS benefit with obinutuzumab was demonstrated for the first time in this study (median OS NR vs 73.1, p=0.0245).
Overall, this data demonstrates a clear benefit with obinutuzumab compared to rituximab with similar tolerability, and as such obinutuzumab is considered the standard anti-CD20 agent in CLL.
Notably, the benefit of obinutuzumab over rituximab has not been consistently demonstrated in other hematologic malignancies
What data is there to support using a first-generation BTK inhibitor in CLL?
The initial data for using covalent BTK inhibitors comes from trials involving ibrutinib-containing regimens: ECOG E1912 and Alliance A041202
ECOG E1912 was a randomized phase 3 trial involving patients younger than 70 with CLL comparing first-line treatment with ibrutinib/rituximab versus FCR.
Based on the most recent updated data with a median follow-up of 6 years, ibrutinib/rituximab had a superior PFS (HR 0.27) as well as a small but statistically significant superior OS (HR 0.47) compared to FCR
Notably, patients with del(17p) were excluded from this trial
Alliance A041202 was a randomized phase 3 trial involving patients older than 65 with CLL comparing first-line treatment with ibrutinib +/- rituximab to BR
Both IR and ibrutinib monotherapy had a superior PFS when compared to BR, however there was no significant difference between IR versus ibrutinib monotherapy.
The latest updated data published in 2024 continues to demonstrate the superior PFS of the ibrutinib-containing arms to BR (median PFS NR in both ibrutinib-containing arms vs 44 months with BR).
Patients with TP53 alterations (deletion or mutation) receiving ibrutinib-containing regimens did not have inferior outcomes compared to those with wild-type TP53 on the same treatment.
What data is there to support using a second-generation BTK inhibitor in CLL?
While ibrutinib is an effective option, there are multiple adverse effects to consider. These include cytopenias (particularly neutropenia), infection, atrial fibrillation, hypertension, and bleeding.
As most patients with CLL tend to be older, these side effects can potentially limit the ability to use ibrutinib safely
Data from the updated E1912 and A041202 trials indicate that 21.9% and 18.1% of patients (respectively) discontinued ibrutinib specifically due to adverse events
As a result, second-generation covalent BTK inhibitors (acalabrutinib and zanubrutinib) have also been studied in CLL (originally in the relapsed/refractory setting, then later in the first-line setting).
Based on experience in CLL and other indolent hematologic malignancies, second generation covalent BTK inhibitors have a more favorable adverse event profile compared to ibrutinib. Patients who do not tolerate ibrutinib may be able to tolerate either acalabrutinib or zanubrutinib without similar issues.
However, they should not be considered as an option for ibrutinib-refractory disease (IE, resistance to ibrutinib confers resistance to either second-generation option).
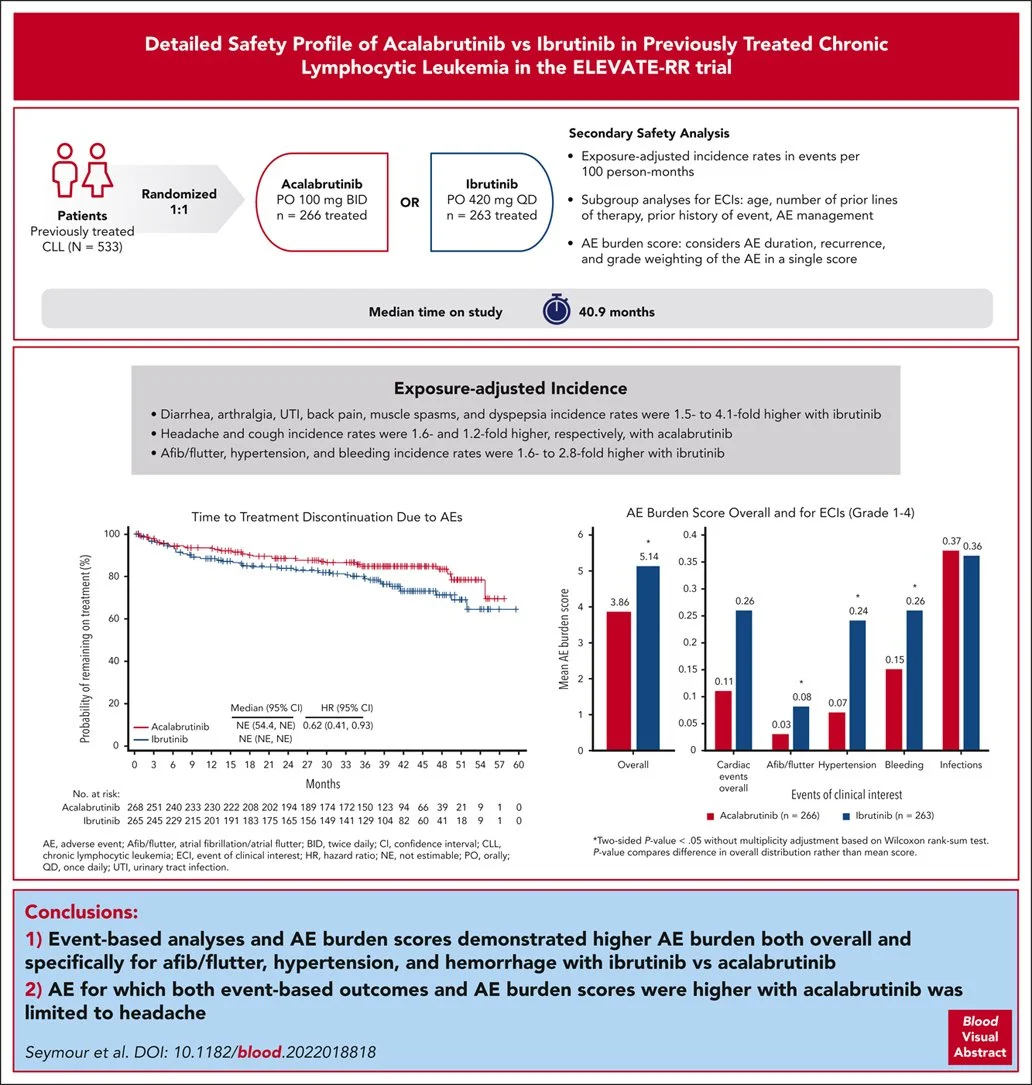
ELEVATE-RR was a randomized non-inferiority phase 3 trial comparing acalabrutinib to ibrutinib in the relapsed/refractory setting (in patients who did not receive a BTK inhibitor as part of first-line treatment).
Acalabrutinib met the pre-specified non-inferiority threshold for PFS, and rates of adverse events (particularly atrial arrhythmias, hypertension, and bleeding) were lower compared to ibrutinib.
Image source: Seymour JF, et al. Detailed safety profile of acalabrutinib vs ibrutinib in previously treated chronic lymphocytic leukemia in the ELEVATE-RR trial. Blood. 2023 Aug 24;142(8):687-699. doi: 10.1182/blood.2022018818.
ALPINE was a randomized phase 3 trial comparing zanubrutinib to ibrutinib in the relapsed/refractory setting (in patients who did not receive a BTK inhibitor as part of first-line treatment) and found evidence of a superior PFS (HR 0.65) with lower rates of cardiac events and lower rates of discontinuing therapy due to adverse events.
Image source: O’Brien. ASH Review Series (2024)
Image source: O’Brien. ASH Review Series (2024)
ELEVATE-TN was a randomized phase 3 trial in patients older than 65 with CLL comparing three arms of treatment: acalabrutinib + obinutuzumab (arm A+O), acalabrutinib monotherapy (arm A), and obinutuzumab/chlorambucil (arm O+Clb)
Based on the most recent updated data presented at ASH in late 2023 (median follow-up 74.5 months), both A+O and A had a superior 6-year PFS rate to O+Clb (78%, 62%, and 18% respectively). A+O was also statistically superior to A (HR 0.58, p=0.0229).
In terms of OS benefit, A+O was statistically superior to O+Clb (6-year OS rate 87% vs 80%; HR 0.62, p=0.0349). Acalabrutinib monotherapy was not superior to O + Clb. A+O trended towards, but did not meet, statistical significance for superior OS compared to A alone (HR 0.69, p=0.1220)
Approximately 12-14% of patients in each arm had TP53 alterations (mutated TP53 and/or 17p deletion). Patients with TP53 alterations continued to show a PFS benefit with A+O and A compared to O+Clb (6-year PFS rate 56%, 56%, and 18% respectively).
Rates of adverse effects with acalabrutinib-containing arms were generally lower compared to historical data with ibrutinib trials, with discontinuation due to adverse events in the original publication estimated at 11% (A+O) and 9% (A) in the acalabrutinib-containing arms. The primary differences in grade ≥3 adverse events seen when adding obinutuzumab to acalabrutinib were neutropenia, infections, diarrhea, and secondary malignancies.
Image source: Sharman JP, et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia. 2022 Apr;36(4):1171-1175. doi: 10.1038/s41375-021-01485-x. Epub 2022 Jan 1. (Note: This is the 4-year follow up study)
SEQUOIA was a randomized phase 3 trial in patients older than 65 with CLL without del(17p) comparing first-line treatment with zanubrutinib vs BR.
Based on the updated data presented in 2023, zanubrutinib demonstrated a superior PFS to BR (median NR vs 42.2 months, HR 0.30) at a median follow-up of ~44 months. Median OS was not reached in either group, but thus far no OS benefit has been demonstrated compared to BR.
A separate cohort of patients with del(17p) was enrolled as part of the SEQUOIA study and these patients also received zanubrutinib. As noted in the updated data, 42 month PFS rates appeared similar to the group without del(17p) (79.4 with del(17p) vs 82.4% with del(17p)).
The side effect profile with zanubrutinib was largely similar to that with acalabrutinib in the ELEVATE-TN study
Image source: Tam CS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022 Aug;23(8):1031-1043. doi: 10.1016/S1470-2045(22)00293-5. Epub 2022 Jul 7. Erratum in: Lancet Oncol. 2023 Mar;24(3):e106
What data is there to support using BCL2 inhibitors in CLL?
The initial data to support the efficacy of venetoclax (a BCL2 inhibitor) in CLL came from the MURANO study, a randomized phase 3 trial that compared venetoclax/rituximab to BR in the relapsed/refractory setting and found both a PFS and OS benefit.
Data supporting the use of venetoclax in the upfront setting comes from two trials: CLL14 (in patients with comorbidities) and GAIA-CLL13 (in fit patients)
Unlike with BTK inhibitors which are given continuously (until progression or intolerance), venetoclax-based regimens are given for a fixed duration (12 months)
CLL14 was a randomized phase 3 trial published in 2019 evaluating fixed-duration venetoclax/obinutuzumab versus chlorambucil/obinutuzumab as first-line treatment in patients with comorbidities (defined as a Cumulative Illness Rating Score of ≥ 6).
Data from the latest update at a median follow-up of 6 years continued to show the PFS and time-to-next-treatment benefits with venetoclax-obinutuzumab over chlorambucil-obinutuzumab. OS approached but did not fully meet statistical significance (6-year OS rate 78.7% vs 69.2%; HR 0.69, p=0.052)
Notably, patients with TP53 alterations were included in this study and represented ~12% of patients in both treatment arms. While the PFS was better with venetoclax/obinutuzumab in patients with TP53 alterations compared to chlorambucil/obinutuzumab, patients who received venetoclax were more likely to progress if they had a TP53 alteration versus those who did not (HR 3.00) as shown below in data from the 4-year update
Image source: Al-Sawaf O, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020 Sep;21(9):1188-1200.
GAIA-CLL13 was a randomized phase 3 trial in fit patients with CLL that featured four arms: chemoimmunotherapy (FCR), venetoclax/rituximab, venetoclax/obinutuzumab, and venetoclax/obinutuzumab/ibrutinib.
Unlike in the CLL14 trial, patients with TP53 alterations were excluded from this study
The triplet arm and the venetoclax/obinutuzumab arm had superior 3-year PFS rates compared to FCR (90.5% and 87.7%), but the venetoclax/rituximab arm failed to demonstrate a superior PFS over FCR.
Rates of adverse events leading to treatment discontinuation were higher in the triplet group compared to venetoclax/obinutuzumab (12.6% vs 5.7%). Infections were also more common (21.2% vs 13.2%)
Image source: Eichhorst B, et al. First-Line Venetoclax Combinations in Chronic Lymphocytic Leukemia. N Engl J Med. 2023 May 11;388(19):1739-1754. doi: 10.1056/NEJMoa2213093.
How do you select an initial treatment regimen in CLL in the modern-day setting?
For patients with newly diagnosed CLL in the modern-day setting, first-line treatment typically involves either a BTKi-based regimen or a venetoclax based regimen.
There is no direct head-to-head data comparing these two approaches directly. Both options are considered to be equally appropriate choices in general, and the decision on which to choose depends on a variety of individualized factors
BTK inhibitors have a well-known side effect profile including increased infection risk, cardiac arrhythmias, fatigue/myalgias/arthralgias, and increased risk of bleeding.
While these side effects are generally less frequent with second-generation, patients with significant risks for these issues may not be ideal candidates for BTK inhibitors.
Concurrent use of anticoagulation is generally avoided with BTK inhibitors, and warfarin should specifically not be used (and was an exclusion criteria in the ELEVATE-TN study).
The major downside of fixed-duration venetoclax/obinutuzumab is the significant increased risk for TLS.
This regimen should generally be avoided in patients with significant renal dysfunction.
This regimen also requires very close and frequent monitoring during the venetoclax dose ramp-up - while some patients can be treated entirely as an outpatient with multiple per-week clinic visits during the ramp-up, other patients require inpatient hospitalization to carefully monitor for TLS (see below)
Both regimens (but particularly venetoclax) may require dose adjustments due to drug-drug interactions with CYP3A4 inhibitors.
The logistics of delivering treatment is also a significant consideration and should be discussed with patients. BTK inhibitors require continuous treatment until progression/intolerance. Venetoclax/obinutuzumab is a fixed-duration treatment lasting one year, however there are substantially more visits for patients in the first few months (and some patients may require multiple inpatient admissions)
BTK inhibitors are relatively straightforward, and treatment is performed entirely in the outpatient setting. If using obinutuzmab with acalabrutinib, obinutuzumab is only given between cycle 2 and cycle 7 (a total of 6 months).
Patients treated with venetoclax/obinutuzumab require very close monitoring for TLS. The venetoclax manufacturer website has info on how to risk stratify patients based on tumor burden and how to conduct monitoring in both the inpatient and outpatient settings.
If using a BTK inhibitor, a second-generation inhibitor (acalabrutinib or zanubrutinib) is generally preferable based on the decreased rates of side effects and possible superiority (at least when used in the second-line setting, based off data from the ALPINE study) over ibrutinib.
Remember that ibrutinib, acalabrutinib, and zanubrutinib have similar activity in terms of resistance, and progression while on any of these agents indicates that the patient will not respond to any of the other covalent BTK inhibitors either. However, it is appropriate to switch from one covalent BTKi to another if the reason for discontinuation is solely due to adverse effects.
Acalabrutinib can either be given as monotherapy or in combination with obinutuzumab.
While both have superior PFS compared to chemoimmunotherapy, the only OS benefit (compared to chemoimmunotherapy) demonstrated thus far has been when acalabrutinib is given in combination with obinutuzumab.
As of the latest 6-year update of ELEVATE-TN, there is now also a confirmed PFS benefit with acalabrutinib/obinutuzumab over acalabrutinib monotherapy.
If using zanubrutinib, there is no clear data to support the use of adding obinutuzumab at this time.
References:
Eichhorst B, et al; German CLL Study Group (GCLLSG). First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016 Jul;17(7):928-942. doi: 10.1016/S1470-2045(16)30051-1.
Goede V, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014 Mar 20;370(12):1101-10. doi: 10.1056/NEJMoa1313984.
Shanafelt TD, et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N Engl J Med. 2019 Aug 1;381(5):432-443. doi: 10.1056/NEJMoa1817073.
Shanafelt TD, et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood. 2022 Jul 14;140(2):112-120. doi: 10.1182/blood.2021014960.
Woyach JA, et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N Engl J Med. 2018 Dec 27;379(26):2517-2528. doi: 10.1056/NEJMoa1812836. Epub 2018 Dec 1.
Woyach JA, et al. Long-term Follow-up from A041202 Shows Continued Efficacy of Ibrutinib Regimens for Older Adults with CLL. Blood. 2024 Jan 12:blood.2023021959. doi: 10.1182/blood.2023021959.
Seymour JF, et al. Detailed safety profile of acalabrutinib vs ibrutinib in previously treated chronic lymphocytic leukemia in the ELEVATE-RR trial. Blood. 2023 Aug 24;142(8):687-699. doi: 10.1182/blood.2022018818.
Brown JR,et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med. 2023 Jan 26;388(4):319-332. doi: 10.1056/NEJMoa2211582. Epub 2022 Dec 13. PMID: 36511784.
Sharman JP, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020 Apr 18;395(10232):1278-1291. doi: 10.1016/S0140-6736(20)30262-2.
Sharman JP, et al. Acalabrutinib ± Obinutuzumab Vs Obinutuzumab + Chlorambucil in Treatment-Naive Chronic Lymphocytic Leukemia: 6-Year Follow-up of Elevate-TN. Blood 2023; 142 (Supplement 1): 636. doi: https://doi.org/10.1182/blood-2023-174750
Sharman JP, et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia. 2022 Apr;36(4):1171-1175. doi: 10.1038/s41375-021-01485-x. Epub 2022 Jan 1.
Tam CS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022 Aug;23(8):1031-1043. doi: 10.1016/S1470-2045(22)00293-5. Epub 2022 Jul 7. Erratum in: Lancet Oncol. 2023 Mar;24(3):e106.
Shadman M, et al. Zanubrutinib vs bendamustine + rituximab in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma: extended follow-up of the SEQUOIA study. Presented at: 17th International Conference on Malignant Lymphoma; June 13-17, 2023. Lugano, Switzerland. Abstract 154.
Fischer K, et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N Engl J Med. 2019 Jun 6;380(23):2225-2236. doi: 10.1056/NEJMoa1815281.
Al-Sawaf O, et al: Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia. EHA2023 Hybrid Congress. Abstract S145. Presented June 8, 2023.
Al-Sawaf O, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020 Sep;21(9):1188-1200.
Eichhorst B, et al. First-Line Venetoclax Combinations in Chronic Lymphocytic Leukemia. N Engl J Med. 2023 May 11;388(19):1739-1754. doi: 10.1056/NEJMoa2213093.
The crew behind the magic:
Show outline: Vivek Patel
Production and hosts: Ronak Mistry, Vivek Patel, Dan Hausrath
Editing: Resonate Recordings
Shownotes: Megan Connor, Sean Taasan
Social media management: Ronak Mistry
We are proud to partner with HemOnc.org!
Want to learn more about the trials that lead to the regimens discussed today? What about dosing schedules? See links in the show notes for a link to HemOnc.org