Episode 072: Introduction to Diffuse Large B-Cell Lymphoma (DLBCL)
This week, we kick off a new series focusing on diffuse large B-cell lymphoma. In this first episode, we discuss the basics that everyone needs to understand before diving into the management of this disease.
This episode has been sponsored by HemOnc.org.
If you have not done so, we highly recommend you listen to our Hemepath series before proceeding with this DLBCL series
How do you approach a patient with concerns for lymphoma?
History: inquire about “B-symptoms” including fevers, drenching night sweats (requiring changing of clothes or sheets), unexpected weight loss, lymphadenopathy
Exam: Assess for lymphadenopathy, examining the neck, axilla, and inguinal lymph nodes
Initial labs: CBC, CMP, LDH
Biopsy:
As we said in our prior episodes, lymphomas require AT LEAST a core biopsy and ideally an excisional biopsy to be able to evaluate the architecture of the lymph node
HOWEVER, do not underestimate the role that a fine needle biopsy (FNA) can play.
An FNA is often a lot faster to get and using flow cytometry and IHC, we can quickly assess if the findings are consistent with a lymphoma or something else. This is especially helpful in cases where other cancers are on the differential - is this metastatic disease of someone’s known malignancy or a new cancer?
FNA or fine needle aspirate is a biopsy technique that gives us a small sample of cells to analyze. It can easily diagnose a solid tumor malignancy. Not ideal for final diagnosis of lymphoma because we need the lymph node architecture.
Bottom line is that we are mainly looking at the phenotype of the cells
What are the characteristic patterns of DLBCL on flow cytometry?
Then flow cytometry helps further define the phenotype of these cells by looking a cell surface protein expression (i.e. CD markers)
CD10 and CD20 are associated with B cells that are germinal center in origin (more to come on this topic soon)
Increased forward scatter on flow tells us that the cells are larger
Increase side scatter on flow would tell us that the cells have high granularity
What are the initial steps for diagnosis and staging of DLBCL?
Imaging:
Always get a PET/CT for staging if possible
If patient is inpatient and PET/CT is not possible, a CT neck, chest, abdomen, pelvis can be sufficient to expedite a work up in a patient who is sick
Viral studies:
Hepatitis B - DLBCL often employs use of anti-CD20 monoclonal antibody, rituximab, which can cause hepatitis B-reactivation, so we want to know patient’s status
If Hep B positive, we start treatment for it concurrently
HIV
Labs:
CBC
CMP
LDH is a very important baseline prognostic marker
Biopsy:
Must get an excisional or core lymph node biopsy for definitive diagnosis. Excisional is always ideal in the case of lymphoma
Do NOT wait for imaging if patient has palpable and accessible lymph nodes in the neck, axilla, and groin; it is very reasonable to ask surgery colleagues for excisional biopsy from what you can palpate
Sometimes an excisional biopsy is not feasible and in those cases a core biopsy can be adequate.
Example: Mediastinal
You do run the risk of a non diagnostic sample but again this is a reasonable approach
How do we risk stratify a patient with DLBCL?
Cell-of-origin classification
History lesson:
There are so many different types of lymphoma and at first we had Thomas Hodgkin discover Hodgkin Lymphoma in the 1800’s
We found out that there were over 61 other types of lymphoma by the 1950s that each had distinct characteristics from Hodgkin Lymphoma and these were lumped into the category of “Non Hodgkin Lymphoma”
The next big step as we do in oncology was risk stratification which was initially primarily done through histological findings and tumor grade
We then looked at gene expression profiling to identify prognostic subgroups and there as a study published in Nature in 2000 that identified three groups:
Germinal center B cell like (GCB), Activated B cell like (ABC), or type 3 gene expression profile
GCB had the best prognosis
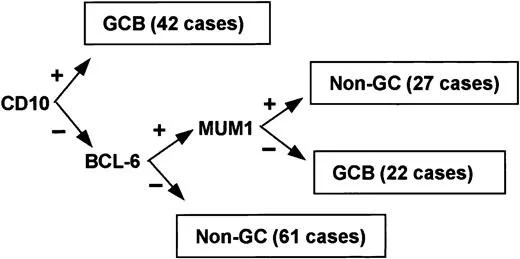
Sending off molecular DNA testing on all biopsies was not cost effective or feasible on a large scale. Christine Hans who is a pathologist from the University of Nebraska published a study that found IHC surrogates to the gene expression profiles. This is called the Hans algorithm
Paper was published in Blood 2003
Developed an algorithm to identify GCB vs. non GCB subtypes
Just as good as DNA microarray for risk stratification and much more cost effective
This is a prognostic but not a predictive marker so it does not necessarily change our management
Key points from this algorithm
CD10+ is germinal center origin
This marker also helps us form a differential for diagnosis of Non-Hodgkin B Cell Lymphoma
Whenever you see CD10+, think DLBCL, follicular lymphoma, or Burkitt lymphoma
BCL6+ is often GCB but not always
BCL6+ and MUM1+ then ABC subtype
I remember “my MUM taught me the ABC’s”
Bottom line: look it up but know that CD10+ helps form a differential diagnosis and BCL6+ is often GCB in origin so common in follicular lymphoma
Image source: Figure 1. Hans et al. Blood (2004).
Genetic changes:
Informs the idea of “double expressor” and “double/triple hit”
Three things to remember are MYC, BCL-2, and BCL-6
MYC:
Helps cells enter S-phase and divide rapidly
HIGH YIELD: MYC is always found on chromosome 8
Think of MYC like the 8 Ball in Pool - the most important ball in the game
Chromosomal rearrangements in 8 important and are high risk → MYC rearranged
BCL-2:
Anti-apoptotic protein so if it has increase activation or is overexpressed then think possibly a more aggressive large B cell lymphoma
Found on chromosome 18 and has a consistent translocation partner, so you will see t(14;18)
BCL-6
Downregulates p53, MYC, and BCL-2 so a rearrangement can be an issue; downregulating p53 is bad (but downregulating BCL-2 and MYC is good).
This is found on Chromosome 3; remember that BCL-6 represses 3 proteins! The translocation partner is inconsistent.
Cytogenetics: Most important aspect
Remember - this is looking at the things like translocations, additions, inversions, deletions using FISH
“Double hit”:
Rearrangement in the most important chromosome 8 (the 8 ball) = MYC AND EITHER BCL-2 or BCL-6
Note: “Additional copies” is not the same as rearrangements!
“Triple hit”:
Rearrangement in MYC, BCL-2, and BCL-6
These are by far the worst prognosis and knowing this will change management for patients without localized disease
IHC:
This is how we determine if someone is “double expressor”
Defined by overexpression on IHC of >50% in MYC and BCL-2
Poor prognosis in several retrospective studies
This is not as important as the cytogenetics
Debatable on whether to change management in double expressor and many would not escalate to more intensive regimens
A key point: BOTH double hit and double expressor will get CNS prophylaxis
Does Ki-67 change management?
High Ki-67 tells us we have a more proliferative lymphoma but it has significantly less prognostic significance than things like clinical stage, extranodal involvement, and cytogenetics
One thing every hematologist/oncologist should know is that a Ki-67 of 100% should be presumed Burkitt Lymphoma until proven otherwise
Can’t diagnose Burkitt Lymphoma unless you also have a rearrangement in the 8 ball, MYC
These patients would not be treated with R-CHOP or Pola-R-CHP but more intensive regimens
These patients will need CNS prophylaxis so it greatly changes management
How does staging work in DLBCL?
Historical context:
You will hear Ann Arbor and Lugano Staging
Ann Arbor staging is the older and more outdated system that was developed prior to PET/CT
When PET/CT started to be used, we actually saw that ~30% of patients with lymphoma were upstaged compared to CT imaging
A group met in Lugano, Switzerland in 2007 to develop the “Lugano Staging”
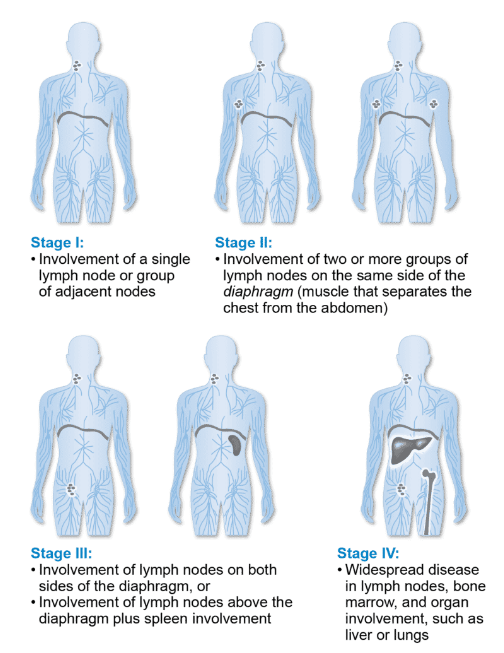
Lugano Staging:
Stage I or II = early stage
Single nodal group or single extranodal site is stage I
Multiple nodes on same side of diaphragm is stage II
Stage III or IV = advanced stage
Nodes above and below the diaphragm is stage III
Diffuse involvement with one or more extranodal site (i.e liver, lung, bone, or bone marrow)
Staging has important implications on how we treat our patients!
NOTE: You do not need to perform a bone marrow biopsy in staging as stage III and IV are treated the same way and are lumped in the same group for prognostication
Image source: https://lymphoma.org/understanding-lymphoma/diagnosing-lymphoma/staging-and-prognosis/
How do we risk stratify our patients?
IPI-score (MDCalc: https://www.mdcalc.com/calc/3936/international-prognostic-index-diffuse-large-b-cell-lymphoma-ipi-r-ipi)
Remember the mnemonic APLES
Age
Performance status
LDH
Extranodal disease (more than one site)
Stage (III or IV)
This episode has been sponsored by HemOnc.org.
References
https://pubmed.ncbi.nlm.nih.gov/10676951/ : Nature publication classifying DLBCL into subtypes
https://ashpublications.org/blood/article/103/1/275/17580/Confirmation-of-the-molecular-classification-of : Hans classification
https://ascopubs.org/doi/10.1200/JCO.2011.41.4342?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed : Publication highlighting treatment implications of sub-classifications of DLBCL
The crew behind the magic:
Show outline: Vivek Patel
Production and hosts: Ronak Mistry, Vivek Patel, Dan Hausrath
Editing: Resonate Recordings
Shownotes: Ronak Mistry
Social media management: Ronak Mistry
We are proud to partner with HemOnc.org!
Want to learn more about the trials that lead to the regimens discussed today? What about dosing schedules? See links in the show notes for a link to HemOnc.org