Episode 099: Colorectal Cancer Series, Pt. 1 - Intro to Colorectal Cancer
Colorectal cancer is one of the most common cancers diagnosed each year worldwide. This highly anticipated series will take an in-depth look at this disease. In this first episode, we discuss the basics, including staging, tumor markers, and microsatellite instability testing, before tackling the management of colorectal cancer in upcoming episodes.
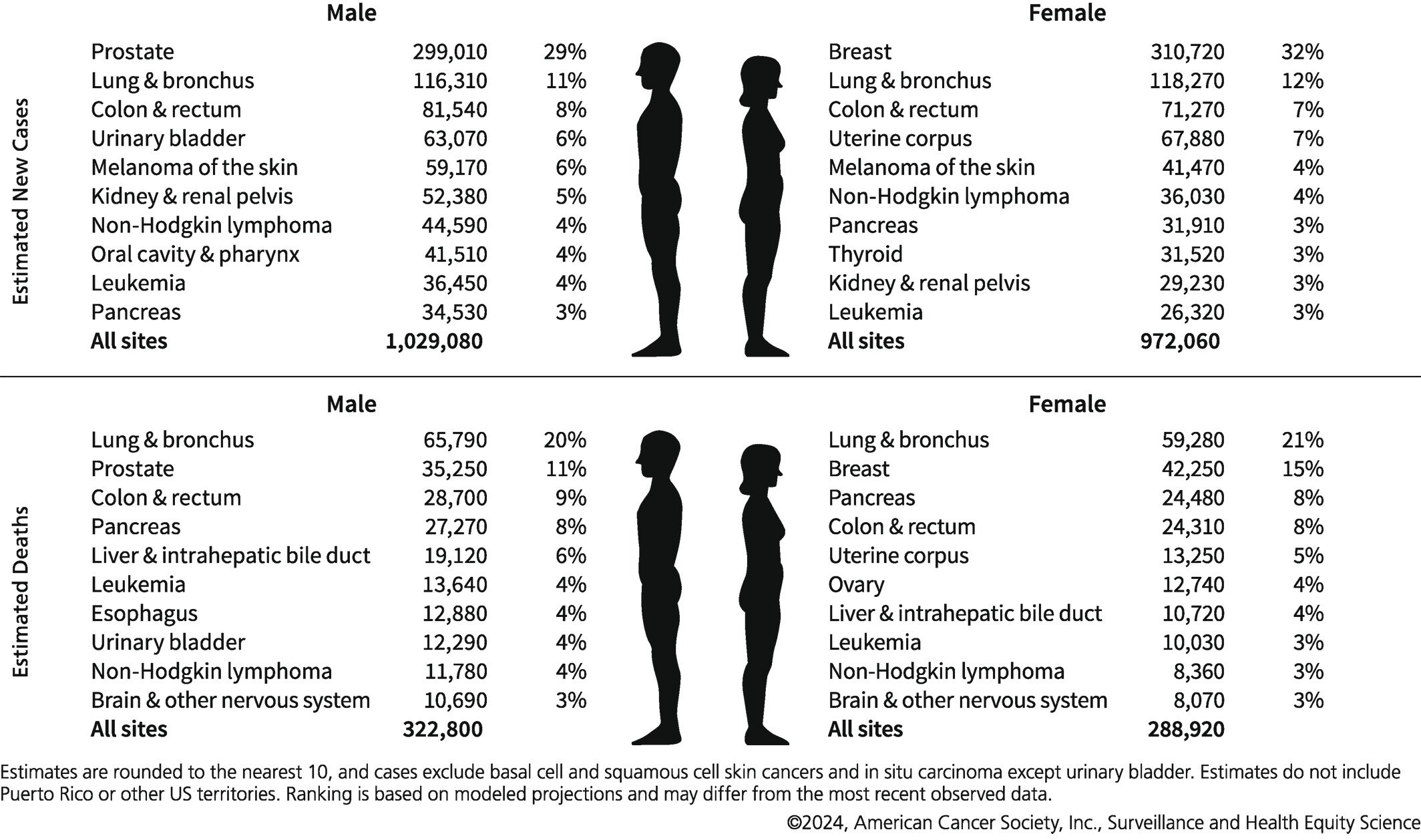
What is the incidence of colorectal cancer in the US?
Colorectal cancer is the third most common cancer in the United States in terms of new cases diagnosed annually
It is third (men) and fourth (women) in terms of cancer-related deaths
Image Source: Seigel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA. https://doi.org/10.3322/caac.21820
What are the screening guidelines for colorectal cancer?
Briefly, screening colonoscopy is recommended for all adults at the age of 45 in the United States per the 2024 USPSTF guidelines
More on screening in a future episode! Also, consider checking out a review of the updated guidelines by our friends, The Curbsiders (Episode #283).
What is the trend regarding incidence of colorectal cancer?
Incidence is on the rise in young adults and is the leading cause of death for men under the age of 50 and second leading cause of death for women under the age of 50
There is increasing incidence in people in their 20s and 30s
Look out for a future episode about this!
Is colorectal cancer treated the same whether it starts in the colon or the rectum?
The treatment of metastatic colon cancer and treatment of metastatic rectal cancer are essentially the same
However, the treatment for colon cancer and rectal cancer are very different in the localized setting! Early-stage colon cancer and early-stage rectal cancer are two biologically different diseases.
How are patients with colorectal cancer staged?
All patients should undergo a staging CT chest/abdomen/pelvis to rule out metastatic disease
If liver lesions are present, obtain an MRI abdomen to better characterize
Lack of widespread availability of MRI greatly affects how we think about appropriate staging in older studies (prior to the late 2000s)
We may have been understaging patients
PET/CT as a limited role in initial staging
During surveillance, PET/CT can be helpful to identify low burden isolated metastatic disease in a patient with a rising CEA
What is synchronous metastatic disease vs. metachronous metastatic disease?
Synchronous metastatic disease means a metastatic lesion that is discovered at the time of initial diagnosis
Metachronous metastatic disease refers to the appearance of metastatic disease during follow-up after treatment of localized disease
Why is metastatic disease in colorectal cancer different?
One important advance in colorectal cancer is definitive treatment for limited metastatic (“oligometastatic”) disease with curative surgical resection followed by adjuvant chemotherapy
Early identification of synchronous metastatic disease is critical to cure these patients
Historically, patients with indeterminate liver lesions were often deemed to not have synchronous metastatic disease
May have undergone surgical resection and were later found to have metachronous metastatic disease
Potentially had a worse disease free survival and overall survival
What is the role of carcinoembryonic antigen (CEA) in colorectal cancer?
Discovered by two Canadian scientists in 1965 and found in malignant tumors of the GI tract epithelium but also in fetal gut and liver (hence “carcinoembryonic”):
Theory is that CEA is repressed during normal GI tract cell differentiation but subsequently reappears in malignant cells due to abnormal maturation
As a result, CEA can be a prognostic marker preoperatively and can also be helpful to detect asymptomatic recurrence in surveillance, following definitive treatment
How does elevated preoperative CEA influence treatment?
In short, it does not change management
However, it does identify CEA as a useful marker for surveillance postoperatively
What is the prognostic significance of CEA?
A few several retrospective studies have examined the prognostic significance of preoperative CEA
One Korean single center study published in 2010 studied 474 patients:
Elevated CEA (defined as > 5) was significantly associated with worse disease free survival and overall survival in patients with stage II colorectal cancer (but not stage III, or node-positive, colorectal cancer)
Despite being shown to be prognostic, CEA has not been used as a predictive tool to help decide whether patients should receive adjuvant chemotherapy
Just because CEA is prognostic (an elevated CEA portends a worse prognosis) does not mean it is predictive (it is not clear that all patients with elevated CEA will benefit from chemotherapy)
Another concern is whether patients with stage II colorectal cancer and elevated CEA were staged properly, either due to lack of thorough lymph node assessment or due to inadequate imaging - this will be a common theme later!
What are microsatellites and the mismatch repair proteins?
Microsatellites are repeating sequences of DNA that consist of a short motif of nucleotides repeated several times
They are found throughout the genome and have high variability among individuals
These areas also exhibit a high mutation rate during DNA replication leading to genetic diversity which leads to polymorphisms
Because there are such high mutation rates which can often be mismatched nucleotides, we have repair proteins to fix major mutations that could lead to the development of malignancy; the mismatch repair proteins are MSH2, MSH6, MLH1, and PMS2 (know these!)
Normally, each individual has a consistent length of these microsatellite repeat sequences
If there is a germline loss or acquired loss of the mismatch repair proteins, more mutations accumulate leading to malignancy
Remember that the germline loss is found in Hereditary Lynch Syndrome
The subsequent tumor tends to have a higher mutational burden given changes in these highly mutable regions with a high expression of tumor specific neoantigens that can be recognized by the immune system and predicts a good response to immunotherapy and a lower response to cytotoxic chemotherapy
What is Lynch syndrome?
Lynch syndrome is an autosomal dominant disorder caused by a germline mutation in one of the four mismatch repair genes
Can also be caused by loss of expression of MSH2 due to deletion in the EPCAM gene
How do we test for mismatch repair protein deficiency?
We do an IHC test for MSH2, MSH6, MLH1, and PMS2 (Need an IHC refresher? Check out Episode 004)
If there is an absence of staining then we define those patients as MMR-deficient
This has a ~98% concordance with MSI-high testing (below)
If there is positive staining for all four proteins, then we call it “MMR proficient”
What is microsatellite instability (MSI) and how do we test for that?
MSI testing is PCR-based testing where we are comparing the length of microsatellites found in the normal tissue compared to the length found in the tumor tissue
Is there instability in the length of the microsatellites in the tumor?
Uses primers for common microsatellite sequences and looks for discrepancy in lengths
Early on, there was no standardization of which primers to use, which led to significant variability in MSI testing
A 1998 National Cancer Institute conference promoted consistency with a standard 5 primer test
If 2 of the 5 primers (≥ 40%) showed different lengths of the microsatellite regions, then the tumor was deemed MSI high (MSI-H)
If one of the primers (< 40%) showed different lengths, then it was deemed MSI low (MSI-L)
If no primers had different lengths, then it was deemed microsatellite stable (MSS)
Eventually, it was shown that MSI low and MSS tumors behaved the same way
MSI high was concordant with loss of mismatch repair proteins and with higher tumor mutational burden
Thus, MSI low (and MSS) = pMMR and MSI high = dMMR
Testing has improved significantly and MSI status is typically listed on next generation sequencing reports
For more on MSI testing, see this review
What is the role of genetic testing in patients with colorectal cancer? Which patients with colorectal cancer should undergo testing for microsatellite instability (MSI)?
Every patient with colorectal cancer (especially under the age of 50) should meet with a genetic counselor to discuss germline testing
All patients with colorectal cancer should either get MSI testing by PCR or mismatch repair (MMR) status by immunohistochemistry
A pivotal study published in JAMA Oncology in 2017 evaluated 450 patients younger than 50 as part of the Ohio Colorectal Cancer Prevention Initiative
All patients underwent germline testing for 25 cancer susceptibility genes and MMR IHC testing
16% of patients were found to have a germline mutation and 10% of patients were found to be MMR deficient
Of the 72 patients with a germline mutation, one third of those patients would not have meet criteria for germline genetic evaluation by consensus guidelines
Another finding was that MMR IHC was nearly 100% concordant with MSI-high PCR testing
The study helped make it clear that all patients younger than 50 should be offered screening for germline testing with a genetic counselor
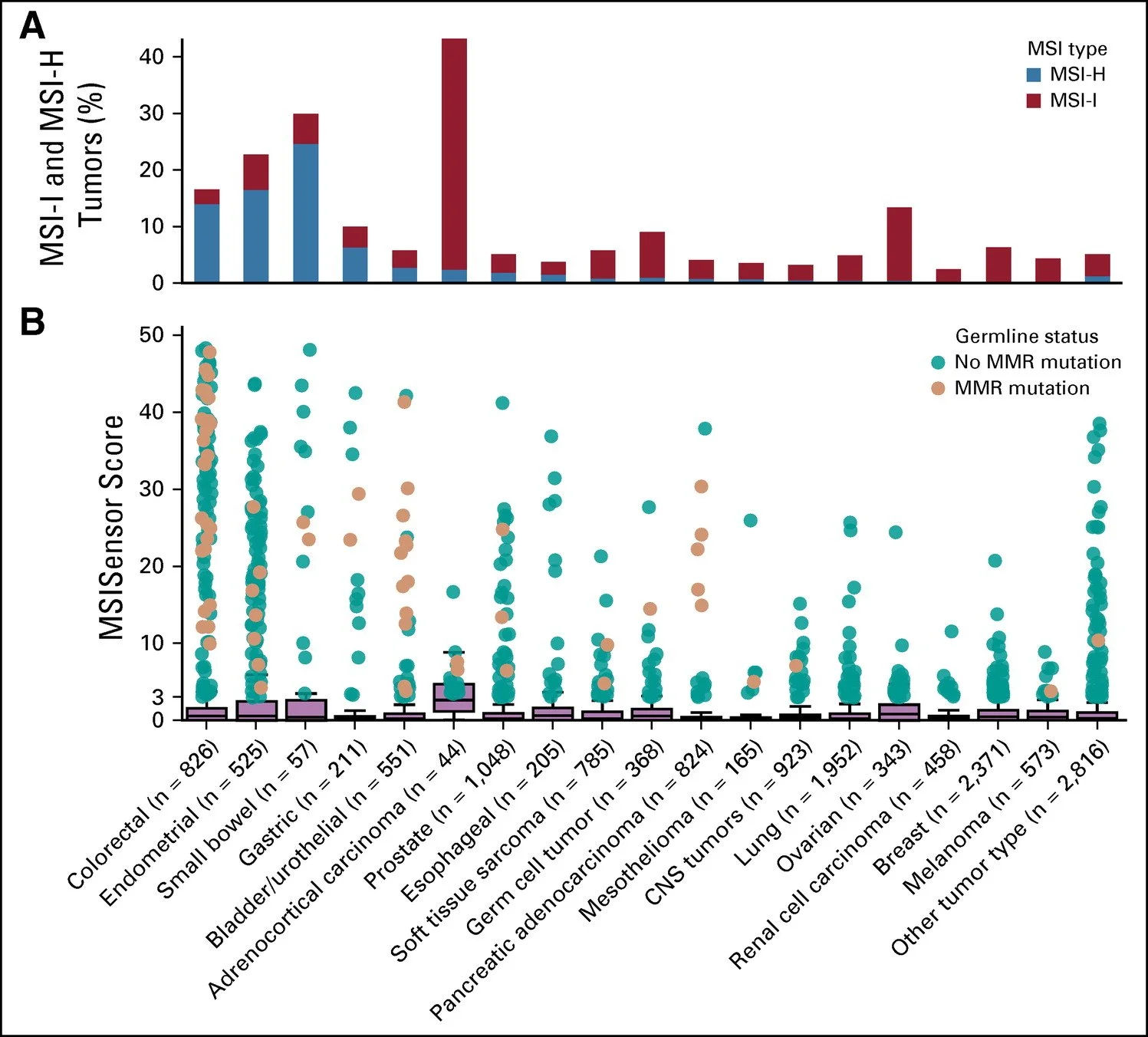
There was another study done at Memorial Sloan Kettering in 2018 was looking at the prevalence of MSI-high tumors across a variety of cancers regardless of age published in JCO 2018
Over 15,000 patients were enrolled encompassing 50 cancer types from 2014-2017
MSI status was determined by a computational algorithm called MSIsensor
Compared reads at designated microsatellite regions in tumor and normal tissue pairs - more sophisticated and precise than just using 5 primers
The most common tumors with MSI-high were small bowel, colorectal, and endometrial (Lynch syndrome!)
Over 800 patients had colorectal cancer and 15% of those patients were found to be MSI-high which is why we recommend all patients undergo this testing
Typically, these tumors tend to be right sided as well
Image source: Figure 1 from Pearlman et al. 2017. JAMA Oncology. https://jamanetwork.com/journals/jamaoncology/fullarticle/2593042
What are the critical components for TNM staging?
T stage is based on depth of invasion rather than size and here’s what you need to know
T2 is invasion into the muscle layer
T3 is extension through the muscle into the pericolorectal tissue
T4a is invasion of the visceral peritoneum (including perforation of the bowel) and T4b is invasion of adjacent organs/structures (i.e. the abdominal wall)
What matters is invasion through the muscle not just into the muscle unlike bladder cancer
What also matters is T4b which is invasion of adjacent organs or other structures
T3 and above is stage 2 assuming no lymph node involvement
N stage is based on the number of nodes found after resection
An adequate surgical resection is at least 12 nodes examined
N1: < 4 nodes
N2: ≥4 nodes
If you have any N, the patient is Stage III regardless of T stage and needs adjuvant chemotherapy
Image source: Figure 1. Chang et. al. 2023. Radiologic T staging of colon cancer: renewed interest for clinical practice. Abdominal Radiology. https://link.springer.com/article/10.1007/s00261-023-03904-2
You may have heard that in the past some stage II patients have worse outcomes than stage III patients. How is this possible?
The evidence behind this concept comes from a study published by Gunderson and colleagues in the JCO in 2009
They looked at the SEER database from 1992-2004 and included nearly 110,000 patients with colorectal cancer
The study found vastly different risks by various T and N combinations with notably T4 and N2 patients having the worst survival
Interestingly, T4bN0 (i.e. invasion to adjacent structures but no nodes) had a 5 year overall survival similar to patients with T3N2 disease (i.e. just through the muscle layer into the pericolorectal tissue and 4 or more nodes)
It is likely that many of these patients had synchronous metastatic disease or actually had nodal involvement if they had modern staging imaging and surgical techniques
Only half the patients in the study had an appropriate number of lymph nodes examined (12 or more) with many having 7 or less examined
This means that some of those T4bN0 patients may have actually had nodal involvement or even metastatic disease
Keep this in mind for when we talk about adjuvant therapy!
What is stage migration?
Stage migration refers to the reclassification of stages in cancer patients, either when the staging system is changed or upon widespread availability of a newer, more accurate diagnostic test
With respect to older colorectal cancer studies, stage migration is a particular concern as newer technologies such as MRI or modern surgical techniques such as higher lymph node retrieval could have reclassified Stage II (node-negative) tumors into Stage III or even Stage IV tumors
A related concept is the “Will Rogers phenomenon” - based on a joke Rogers made in the 1930s regarding migration in the Great Depression
“When the Okies left Oklahoma and moved to California, they raised the average intelligence level in both states!”
In other words, reclassifying patients from one stage to a higher stage (for example, due to MRI being more sensitive for metastatic lesions) might actually improve the prognosis in both stages - while the highest-risk patients removed from the lower stage, those patients typically have a prognosis that is not quite as poor as the majority of patients in the higher stage
What are other important features to be aware of on the pathology report?
If any of these are met, we tend to think of these patients as having higher risk of disease recurrence regardless of nodal involvement
Patients need at least 12 lymph nodes examined
Reviewing less than 12 may mean that we are misclassifying them.
Poorly differentiated histology
Perineural invasion
Vascular invasion
Obstruction or perforation at the time of surgical resection
Where did these risk factors come from?
These risk factors were derived from several retrospective studies conducted in the 1990s to 2010.
Why is this important in 2024?:
We have improvements in imaging and surgical techniques now. Were we understanding patients before? Were we missing metastatic disease?
MSI/MMR testing is more routine now than it was then. How does this play a role?
This matters because these risk factors are often used when considering who to treat with adjuvant therapy. But we should not be overly dogmatic and need to have a discussion. More on this in future episodes.
References:
https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21820: Cancer statistics 2024 report
https://rupress.org/jem/article/122/3/467/3878/SPECIFIC-CARCINOEMBRYONIC-ANTIGENS-OF-THE-HUMAN : Story of CEA
https://onlinelibrary.wiley.com/doi/abs/10.1002/jso.21495: Retrospective analysis looking at CEA as a risk factor for curative colon cancer
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2903331/ : JCO 2010 article discussing the importance of MSI testing in colorectal cancer
https://jamanetwork.com/journals/jamaoncology/fullarticle/2593042: JAMA Oncology 2017 article discussing the need for germline testing in young patient with colorectal cancer
https://ascopubs.org/doi/10.1200/JCO.2009.24.0952?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed#F1: Article discussing high risk pathologic features for colon cancer
The crew behind the magic:
Show outline: Vivek Patel
Production and hosts: Ronak Mistry, Vivek Patel, Dan Hausrath
Editing: Resonate Recordings
Shownotes: Ronak Mistry, Neil Biswas
Social media management: Ronak Mistry
We are proud to partner with HemOnc.org!
Want to learn more about the trials that lead to the regimens discussed today? What about dosing schedules? See links in the show notes for a link to HemOnc.org
Quick reminders:
Mismatch repair proteins: MSH2, MSH6, MLH1, and PMS2
MSI-low = pMMR; MSI-high = dMMR [more mutations]