Episode 100: Colorectal Cancer Series, Pt. 2 - Adjuvant Therapy in Stage III Colon Cancer
This week, we kick off our discussion of adjuvant systemic treatment in colon cancer, beginning with Stage III colon cancer. We will review the evidence basis for adjuvant therapy as well as the two main chemotherapy regimens including duration and side effects.
How does the TNM staging system work again?
Check out the previous episode for a refresher
Stage II colorectal cancer means at least T3 (but no lymph node involvement)
Stage III colorectal cancer means nodal involvement
In stage III colorectal cancer, the highest risk group is T4 and/or N2
Why is leucovorin given with 5-fluorouracil (5-FU)?
Leucovorin increases the enzymatic binding of 5-FU
Likely most useful when using bolus 5-FU rather than 48-hour infusional 5-FU
Many clinicians now feel that bolus 5-FU is not needed prior to infusional 5-FU
More on this in our upcoming Pharmacy episode!
“5-FU monotherapy” implies “with leucovorin” - the monotherapy refers to the lack of an additional chemotherapy agent such as oxaliplatin
What is the evidence basis for adjuvant chemotherapy in colon cancer?
In the 1980s, several studies (such as this one) demonstrated the superiority of 5-FU monotherapy over observation in patients with nodal disease
In 1990, an NEJM study compared 5-FU with levamisole (an antihelminthic drug!) vs. levamisole alone vs. observation
The proposed mechanism was that levamisole had a positive immunomodulatory benefit with upregulation of tumor MHC 1 and upregulation of other parts of the immune system including NK cell activity
There was a 30% benefit in overall survival (OS) for patients with lymph node involvement as compared to surgery alone
As one might have expected, there was no benefit to levamisole monotherapy compared to observation
Subsequent studies from North America and Europe demonstrated the efficacy of 5-FU with leucovorin
Levamisole was subsequently discarded as it did not add efficacy
In the early 2000s, two meta-analyses (JCO and Cochrane) established the likelihood of recurrence in patients who had surgery alone compared to 5-FU monotherapy
The absolute risk of recurrence was about 20% in patients with stage II disease without chemotherapy and 30-40% for stage III without chemotherapy
The absolute risk was decreased by 6-12% in patients with stage III disease who received chemotherapy
Benefit of chemotherapy was much less (1-3%) for patients with stage II disease
The “high risk features” discussed in the previous episode are prognostic variables identified from these older studies and do not have as much significance in stage III disease (compared to stage II disease)
More on adjuvant therapy in stage II colon cancer in the next episode!
What is FOLFOX?
FOL (folinic acid, aka leucovorin) + F (5-fluorouracil) + OX (oxaliplatin)
Kaplan–Meier Estimates of Disease-free Survival in the Group Given Fluorouracil and Leucovorin (FL) and the Group Given FL plus Oxaliplatin, According to the Stage of Disease and the Intention to Treat. Source: André T et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004 Jun 3;350(23):2343-51
How did oxaliplatin come to be used in the adjuvant setting?
Various studies (such as this) had shown that doublet combinations with 5-FU and oxaliplatin were effective in metastatic disease, so trials began to evaluate using this regimen in earlier stages
The MOSAIC trial lead to the approval of FOLFOX for 6 months in patients with stage III colon cancer
Enrolled over 1100 patients with stage II or stage III colon cancer
Patients were randomized to 5-FU monotherapy vs. FOLFOX (given every two weeks) for a total of six months
Important: infusional 5-FU was used instead of bolus 5-FU
There was an absolute benefit of 5% in disease-free survival (DFS) in patients receiving FOLFOX compared to those receiving 5-FU
Most of the benefit was derived from stage III patients
Long-term follow up showed a 4% OS benefit in patients with stage III disease, with a DFS of ~7%
However, there was no OS benefit in stage II disease
Another trial evaluated the use of bolus 5-FU and oxaliplatin but was less convenient and had higher toxicity due to weekly 5-FU infusions for 6 straight weeks
Based on the MOSAIC trial, FOLFOX (with infusional 5-FU) for six months became the standard of care for adjuvant therapy, but only in patients with stage III disease
What is capecitabine and what is its role in colon cancer?
Capecitabine (brand name Xeloda) is an oral prodrug of 5-FU that is given twice daily, 14 days on and 7 days off, in 21 day cycles
Evaluated as monotherapy for patients with stage II disease and combined with oxaliplatin for stage III disease
Leucovorin is not needed to enhance drug activity (as it does for infusional 5-FU)
The XELOXA trial showed that capecitabine + oxaliplatin (CAPOX) had superior DFS vs. 5-FU monotherapy in patients with stage III colon cancer
Another trial (X-ACT) had previously showed that capecitabine had equivalent DFS compared to 5-FU monotherapy
When using chemoradiation in rectal cancer, capecitabine is preferred due to the convenience of oral chemotherapy instead of intravenous 5-FU
However, capecitabine does have more toxicity (higher incidence of diarrhea and hand-foot syndrome) compared to 5-FU
Following MOSAIC, several randomized studies compared FOLFOX and CAPOX alone or with other agents, and other studies evaluated 3 months vs. 6 months duration
What is the incidence of neuropathy with six months of oxaliplatin?
FOLFOX has twelve doses of oxaliplatin, which can be difficult for patients to complete due to neuropathy
Important: Even though CAPOX has only eight doses of oxaliplatin (since it is given every three weeks), the dose intensity is similar because CAPOX utilizes a higher dose of oxaliplatin (130 mg/m2) compared to FOLFOX (85 mg/m2)
One Dutch population-based registry study sent surveys out to all colorectal cancer survivors diagnosed between 2000-2008 about neuropathy and quality of life
Over 1600 patients responded, which was an 83% response rate!
Because the study was conducted in the 2000s, some patients had 5-FU monotherapy and were not exposed to oxaliplatin
The incidence of chronic sensory neuropathy was 30% for patients who received oxaliplatin compared to 8% in patients who received 5-FU monotherapy
This corresponded to a clear reduction in quality of life
The 2015 American ALLIANCE cooperative group study randomized patients to IV magnesium and calcium vs. placebo prior to FOLFOX infusions to prevent neuropathy
There was no benefit to prophylactic therapy
After the completion of 12 cycles of therapy, patients continued to experience neuropathy progression for the next 3 months before seeing some improvement
This phenomenon is known as “coasting”
At the 18 month mark, about 50% of patients still had neuropathy and 30% had severe symptoms
Most patients had persistent sensory neuropathy in their feet, while motor neuropathy was less common
A 2019 Finnish study retrospectively evaluated neuropathy in over 140 patients who got either CAPOX or FOLFOX
There was no difference in neuropathy between CAPOx or FOLFOX
Long term neuropathy was present in 69% of patients
About 25% of patients had grade 2 neuropathy
10% of patients had grade 3 or higher
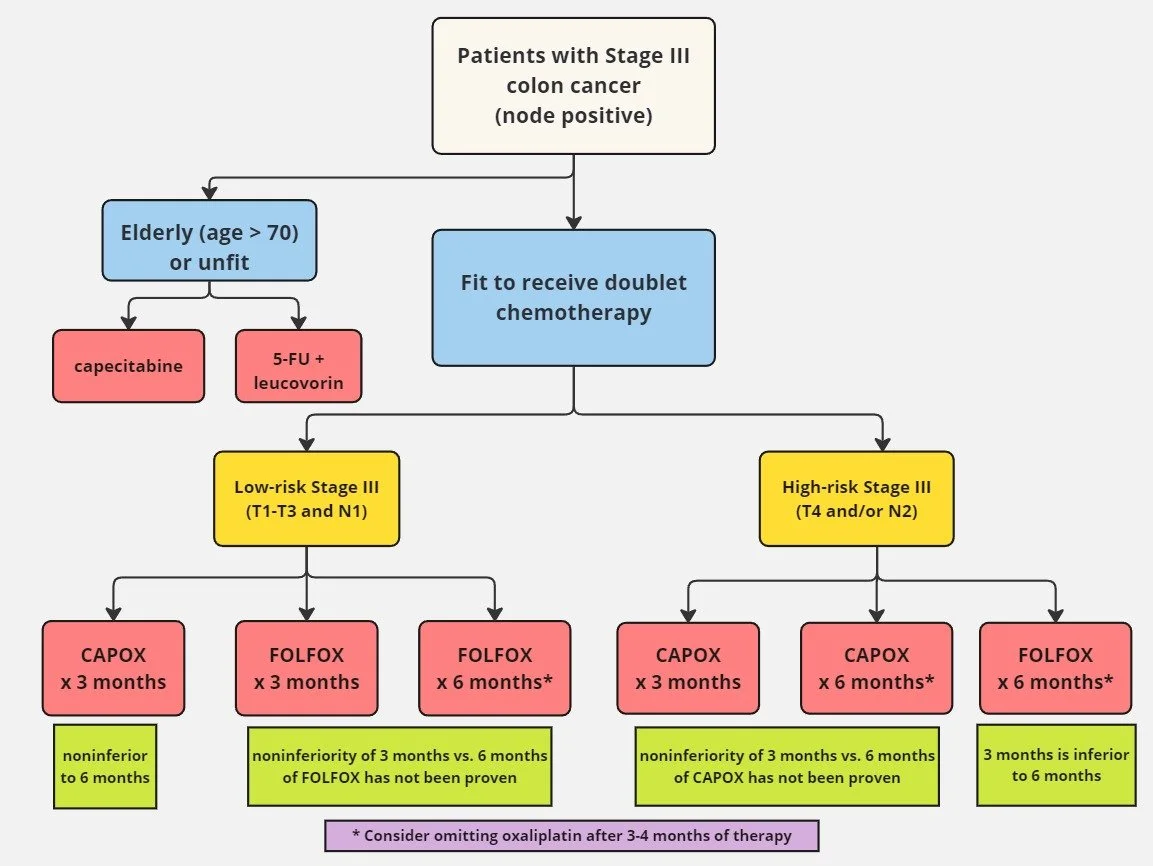
What is the IDEA pooled analysis?
IDEA (International Duration Evaluation of Adjuvant Therapy) was a group of international investigators who sought to determine the optimal duration of adjuvant chemotherapy
Pooled together data from 2007 to 2017 evaluating over 13,000 patients from six separate randomized phase III trials in 12 countries with at least one of the following treatments:
FOLFOX x 3 or 6 months
CAPOX x 3 or 6 months
All other arms in the six trials were excluded in this analysis
Noninferiority trial with a margin (chosen by group consensus) that would tolerate up to a 2.7% margin of loss of efficacy in 3 year DFS
About 60% of the patients received FOLFOX and 40% of the patients received CAPOX
About 3500 patients received 3 months FOLFOX and 3500 received 6 months
About 2500 patients received 3 months CAPOX and 2500 received 6 months
Outcomes were stratified by patient risk based on T and N stage
T1-T3 and N1 was considered low risk
T4 and/or N2 was considered high risk
Other high risk factors (discussed in the previous episode) were not used in this study; again, these are considered more relevant in stage II disease
How did T4 and/or N2 come to be identified as high risk Stage III disease?
A 2010 pooled analysis including older studies from the 1990s and early 2000s evaluated adjuvant 5-FU monotherapy, especially in patients with stage II disease
Showed that MSI-H or dMMR cancers had a worse DFS and OS
The hypothesis was that chemotherapy blunts the immune system and inhibits immune surveillance from eradicating residual disease after surgery
In 2015, a 10 year updated analysis of the MOSAIC trial evaluated predictors of outcomes and specifically outcomes stratified by MMR status (and BRAF mutation)
Unlike the 2010 study, there was a trend towards benefit in DFS and OS seen in patients with dMMR tumors (not statistically significant)
In multivariate analysis controlling for stage, T4 and N2 were both adverse risk factors for DFS and OS
As a result, T4 and/or N2 tumors are considered to be high risk stage III colon cancer
Disease-free Survival with 3 Months versus 6 Months of Therapy, According to Subgroup. Source: Grothey A et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med. 2018 Mar 29;378(13):1177-1188
What are the implications of IDEA on the duration of adjuvant chemotherapy in stage III colon cancer?
There was very little difference in 3-year DFS for all patients when comparing 3 months vs. 6 months with either FOLFOX or CAPOX
3 year DFS was 74.6% for 3 months vs. 75.5% for 6 months, which was not statistically significant for noninferiority
Remember that the threshold of a 2.7% absolute difference was arbitrarily chosen!
The incidence of grade 2 neuropathy one month after treatment was 16% in patients receiving 3 months of treatment compared to almost 50% in patients receiving 6 months
In low risk stage III patients, 3 months of CAPOX was statistically noninferior to 6 months of CAPOX
The absolute 3 year DFS difference for CAPOX was 2% favoring the 3 month group (!)
Why did extending CAPOX to 6 months lead to a lower DFS? Unclear, although some of these differences are unlikely to be clinically meaningful
However, in low risk stage III patients, 3 months of FOLFOX did not meet statistical significance for noninferiority compared to 6 months of FOLFOX
The absolute 3 year DFS difference for FOLFOX was 2.6% favoring the 6 month group
Is this ~2% different clinically meaningful given all the added neuropathy?
Given this data, most clinicians would agree that CAPOX for 3 months should be standard for fit patients with low risk stage III disease
Remember, CAPOX is harder to tolerate than FOLFOX
Can still justify giving FOLFOX for 3 months - but technically, 3 months of FOLFOX was not non-inferior (but not inferior”!) to 6 months in low risk stage III disease
For high risk stage III, absolute 3 year DFS difference for 3 months did not meet noninferiority compared to 6 months, regardless of the regimens
The absolute 3 year DFS difference for CAPOX 3 vs. 6 months was only 0.1%
Nevertheless, this missed the noninferiority margin, possibly owing to the number of patients in the group
The absolute 3 year DFS difference for FOLFOX 3 vs. 6 months was 3.2%
In a subgroup analysis, FOLFOX 3 months was statistically inferior
Based on this data, patients with high risk stage III disease should get 6 months of adjuvant therapy
Why might there be discrepancies between CAPOX and FOLFOX?
There is a possibility that capecitabine is simply a more active drug
When looking at the trials in the IDEA analysis individually:
For two trials that compared 3 vs. 6 months duration, the choice of regimen of FOLFOX or CAPOX was left to the physician
In the US, CAPOX is given to more fit patients given the increased toxicity
Thus, may be selecting for a slightly more favorable patient population when conducting a pooled analysis
There was one CAPOX trial where FOLFOX was not offered
This trial may have enrolled more fit patients, given concern for tolerance
Overall, there is a possibility that CAPOX arms include more fit patients who might have a slightly different disease biology as compared to the FOLFOX arms
The data showing that CAPOX for 3 months is preferred in low risk stage III disease is only an exploratory post-hoc analysis
A 2019 systematic review and meta analysis conducted separately from the IDEA pooled analysis showed that 3 months of CAPOX or FOLFOX was noninferior to 6 months duration
Confirmed the IDEA analysis and showed that the difference between FOLFOX and CAPOX (if there even is one!) is likely small
There is no true comparative efficacy data comparing FOLFOX and CAPOX and overall there is not a drastic difference between these regimens
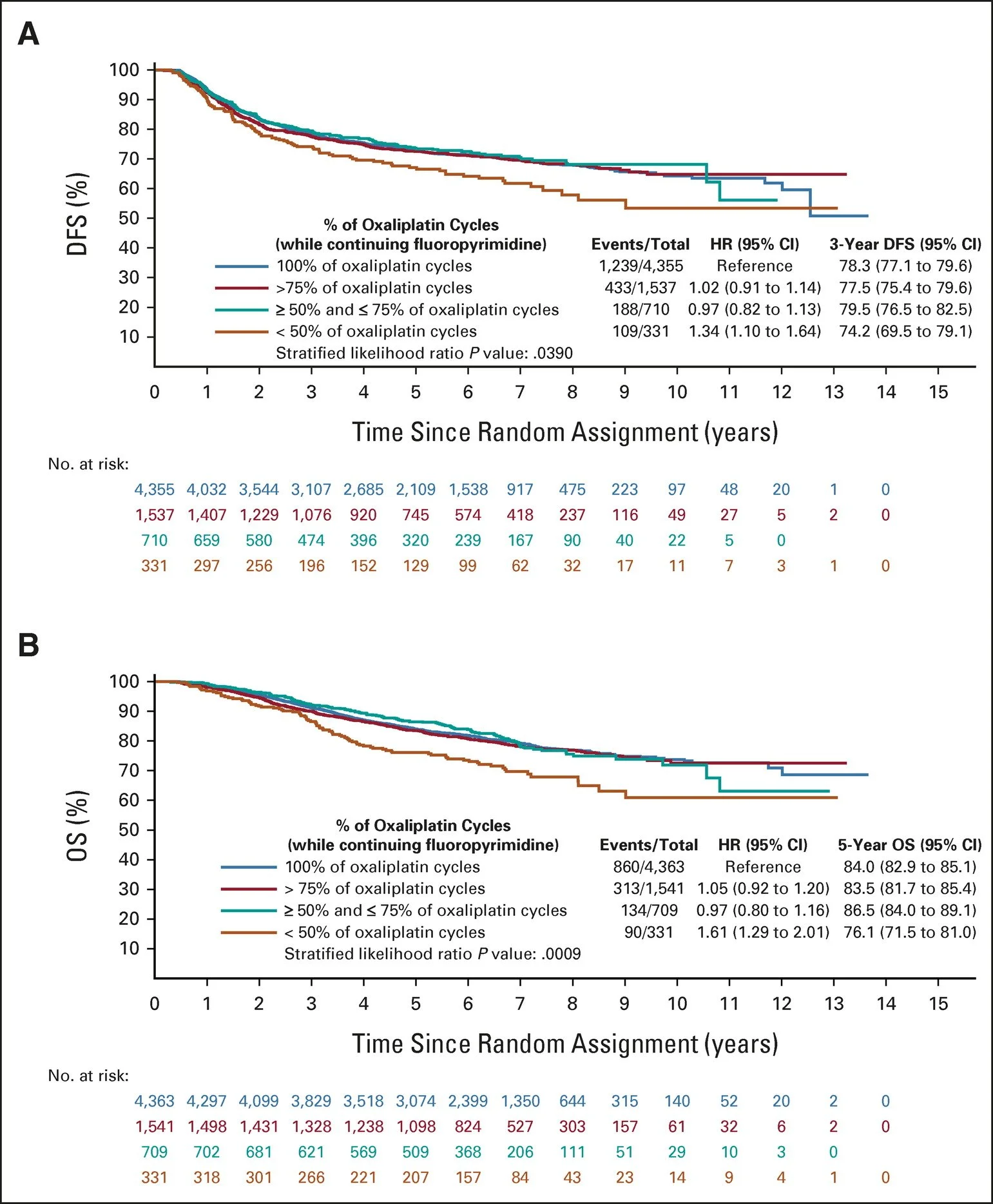
Adjusted Kaplan-Meier curves for OS according to the number of oxaliplatin cycles received. Source: Gallois C et al. Prognostic Impact of Early Treatment and Oxaliplatin Discontinuation in Patients With Stage III Colon Cancer: An ACCENT/IDEA Pooled Analysis of 11 Adjuvant Trials. J Clin Oncol. 2023 Feb 1;41(4):803-815
Is there any data on stopping oxaliplatin early?
Most clinicians stop oxaliplatin after 3 or 4 months of treatment to reduce the risk of chronic neuropathy
A 2023 pooled analysis, including over 10,000 patients from 11 randomized adjuvant colon cancer trials from the ACCENT (Adjuvant Colon Cancer Endpoints) and IDEA databases, evaluated the impact of early discontinuation of all treatment and early discontinuation of oxaliplatin while continuing 5-FU or capecitabine monotherapy
There was no difference in DFS or OS with early oxaliplatin discontinuation as long as more than 50% of the intended doses were delivered
However, there was inferior DFS and OS if all treatment was discontinued early
This confirmed that oxaliplatin may be discontinued after 3 months and patients can be continued on either capecitabine or infusional 5-FU monotherapy, in order to mitigate the risk of long-term neuropathy
Does oxaliplatin have the same benefit in elderly patients?
A recent pooled analysis showed there may be minimal benefit when giving oxaliplatin to patients over the age of 70 with resected colon cancer
Given this, patient fitness should strongly be considered when making decisions regarding adjuvant therapy
For some patients, 6 months of 5-FU monotherapy may be appropriate
How do we put all of this information together?
Image source: Dr. Neil Biswas
References:
https://academic.oup.com/jnci/article-abstract/80/1/30/886027?redirectedFrom=PDF: Older study showing 5-FU better than observation in patients with nodal disease
https://www.nejm.org/doi/full/10.1056/NEJM199002083220602: Older NEJM study investigating levamisole and 5-FU for adjuvant therapy in colon cancer
https://ascopubs.org/doi/10.1200/JCO.2004.09.059: Meta-analysis in JCO investigating likelihood of disease recurrence in colon cancer patients s/p resection
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD005390.pub2/full: Cochrane meta-analysis investigating likelihood of disease recurrence in colon cancer patients s/p resection
https://ascopubs.org/doi/10.1200/JCO.2000.18.1.136: JCO publication from 2000 showing benefit of 5-FU + oxaliplatin in metastatic setting
https://www.nejm.org/doi/full/10.1056/NEJMoa032709: Pivotal MOSAIC trial from NEJM that established FOLFOX as the SOC in stage III colon cancer patients
https://ascopubs.org/doi/10.1200/JCO.2006.08.2974#:~:text=A%20clinical%20trial%20(Multicenter%20International,(LV%3B%20FOLFOX4)%20produced%20a: Study establishing that bolus 5-FU had more toxicity compared to 5-FU infusions
https://ascopubs.org/doi/10.1200/JCO.2006.08.1075: 2007 XELOXA study in JCO showing that capecitabine + oxaliplatin had superior DFS vs. 5-FU monotherapy in stage III colon cancer patients
https://www.nejm.org/doi/full/10.1056/NEJMoa043116: NEJM 2005 X-ACT study establishing capecitabine being equivalent to 5-FU monotherapy
https://ascopubs.org/doi/10.1200/JCO.2013.49.1514?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed: 2013 JCO article assessing neuropathy in colorectal cancer patients receiving oxaliplatin
https://ascopubs.org/doi/10.1200/JCO.2014.58.8533: ALLIANCE cooperative group study JCO 2015 assessing if prophylaxis can decrease neuropathy
https://www.tandfonline.com/doi/full/10.1080/0284186X.2018.1556804#:~:text=Conclusions%3A%20Neuropathy%20following%20oxaliplatin%20containing,treated%20and%20QOL%20is%20similar: A 2019 Finnish study evaluating neuropathy in CAPOX vs. FOLFOX
https://www.nejm.org/doi/full/10.1056/NEJMoa1713709: pivotal IDEA trial evaluating 3- vs. 6-months of CAPOX vs. FOLFOX therapy in stage III colon cancer patients
https://ascopubs.org/doi/10.1200/JCO.2015.63.4238: A 2010 updated analysis of MOSAIC which lead to data suggesting T4 and N2 disease were both adverse risk factors for DFS and OS
https://jamanetwork.com/journals/jamanetworkopen/article-abstract/2733438: A 2019 JAMA article that supported IDEA data
https://ascopubs.org/doi/10.1200/JCO.21.02726?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed: A pooled analysis that suggests that oxiplatin may be discontinued after 3 months of therapy if given with capecitabine or 5—FU
https://ascopubs.org/doi/10.1200/JCO.23.00354: JCO 2023 article suggesting that oxaliplatin should NOT be given to older patients > age 70
The crew behind the magic:
Show outline: Vivek Patel
Production and hosts: Ronak Mistry, Vivek Patel, Dan Hausrath
Editing: Resonate Recordings
Shownotes: Ronak Mistry, Neil Biswas
Social media management: Ronak Mistry
We are proud to partner with HemOnc.org!
Want to learn more about the trials that lead to the regimens discussed today? What about dosing schedules? See links in the show notes for a link to HemOnc.org
Quick reminders:
Mismatch repair proteins: MSH2, MSH6, MLH1, and PMS2
MSI-low = pMMR; MSI-high = dMMR [more mutations]
Staging Reminders!
Image source: Figure 1. Chang et. al. 2023. Radiologic T staging of colon cancer: renewed interest for clinical practice. Abdominal Radiology. https://link.springer.com/article/10.1007/s00261-023-03904-2