Episode 090: Prostate Cancer Series, Pt. 7- Treatment for Met. Castrate Resistant Prostate Cancer
We made it to the end of another series. In our FINAL episode of the prostate cancer series, we turn our attention to metastatic castrate-resistant prostate cancer! We discuss treatment options, the data behind why we do what we do, and more targeted agents.
What are the indications for LHRH antagonists (e.g. degarelix) rather than LHRH agonists (e.g. leuprolide)?
To avoid the testosterone flare that occurs with using an agonist like leuprolide
In inpatient emergencies like spinal cord impingements, always use an antagonist to prevent a flare and worsening of the issue.
In most other situations, testosterone flare can be mitigated by concomitant androgen receptor antagonist therapy
What is classified as high-volume disease?
High-volume disease is defined as visceral metastases or 4 or more bone mets with one outside of the axial skeleton
What is castrate-resistant prostate cancer?
Castrate-resistant prostate cancer is defined as disease increasing in volume or progressing despite testosterone level less than 50 ng/dL.
What tests should be ordered in patients with metastatic prostate cancer?
BRCA mutation germline testing
There are targeted treatment options in the castrate-resistant setting
Mismatch repair gene / microsatellite instability (MSI) testing
Patients could be candidates for immunotherapy if MSI high
PSMA PET scan
If patient has PSMA avid lesions, patient may be a candidate for radioligand therapy
What is the role of bisphosphonate therapy in patients with metastatic castrate-resistant prostate cancer?
Older phase III RCT comparing zoledronic acid (Zometa) to placebo in patients with metastatic castrate-resistant prostate cancer
Enrolled over 400 patients from 1998 to 2002
Primary endpoint was proportion of patients with a skeletal related event (SRE) defined by:
Pathologic bone fracture (didn’t have to be symptomatic)
Radiation to painful bone metastases
Surgery for bone metastasis
Change of systemic therapy for bone pain
Patients were monitored with bone scans at month 6 and 15 then skeletal surveys every 3 months
Reduction in SREs by absolute difference of 11% in zoledronic acid group
Prolonged time to first SRE by about 5 months in zoledronic acid group
Conclusion: Zoledronic acid is effective for reducing skeletal related events in patients with metastatic castrate-resistant prostate cancer
What is the role of bisphosphonate therapy in patients with metastatic castrate-sensitive prostate cancer?
A cooperative group study looking at the utility of zoledronic acid (Zometa) for castrate sensitive patients
Enrolled 650 patients from 2004 to 2012
Same design as prior trial except there was no imaging monitoring requirement
No difference in incidence of skeletal related events or time to first SRE
Conclusion: Zoledronic acid is not effective for reducing skeletal related events in patients with metastatic castrate-sensitive prostate cancer
Therefore, zoledronic acid is only used in the castrate-resistant setting
What dosing schedule is preferred for zoledronic acid?
Every 3 month dosing is non inferior to monthly dosing
This was demonstrated in the following trial, which enrolled patients with breast cancer, prostate cancer, or multiple myeloma
How does denosumab compare to zoledronic acid in patients with castrate-resistant prostate cancer?
Denosumab must be given monthly
There was a phase III RCT that compared denosumab to zoledronic acid including over 1800 patients
Found denosumab had improved time to first skeletal related event by 3 months
Otherwise, there was no improvement in progression free survival or overall survival
There was also higher rates of hypocalcemia in the denosumab arm at 13% vs. 6% in the zoledronic acid arm
There was no difference in osteonecrosis of the jaw, which was roughly 1% in both groups
Given the modest improvement by 3 months and risk of hypocalcemia with more frequent dosing, many clinicians prefer zoledronic acid but both are very reasonable options
Bisphosphonates (e.g. zoledronic acid) preferentially bind to the surface of the bone and provide durable protection as compared to denosumab (a RANK ligand inhibitor) which is effective only as long as it is in the serum and does not have the same “stickiness”
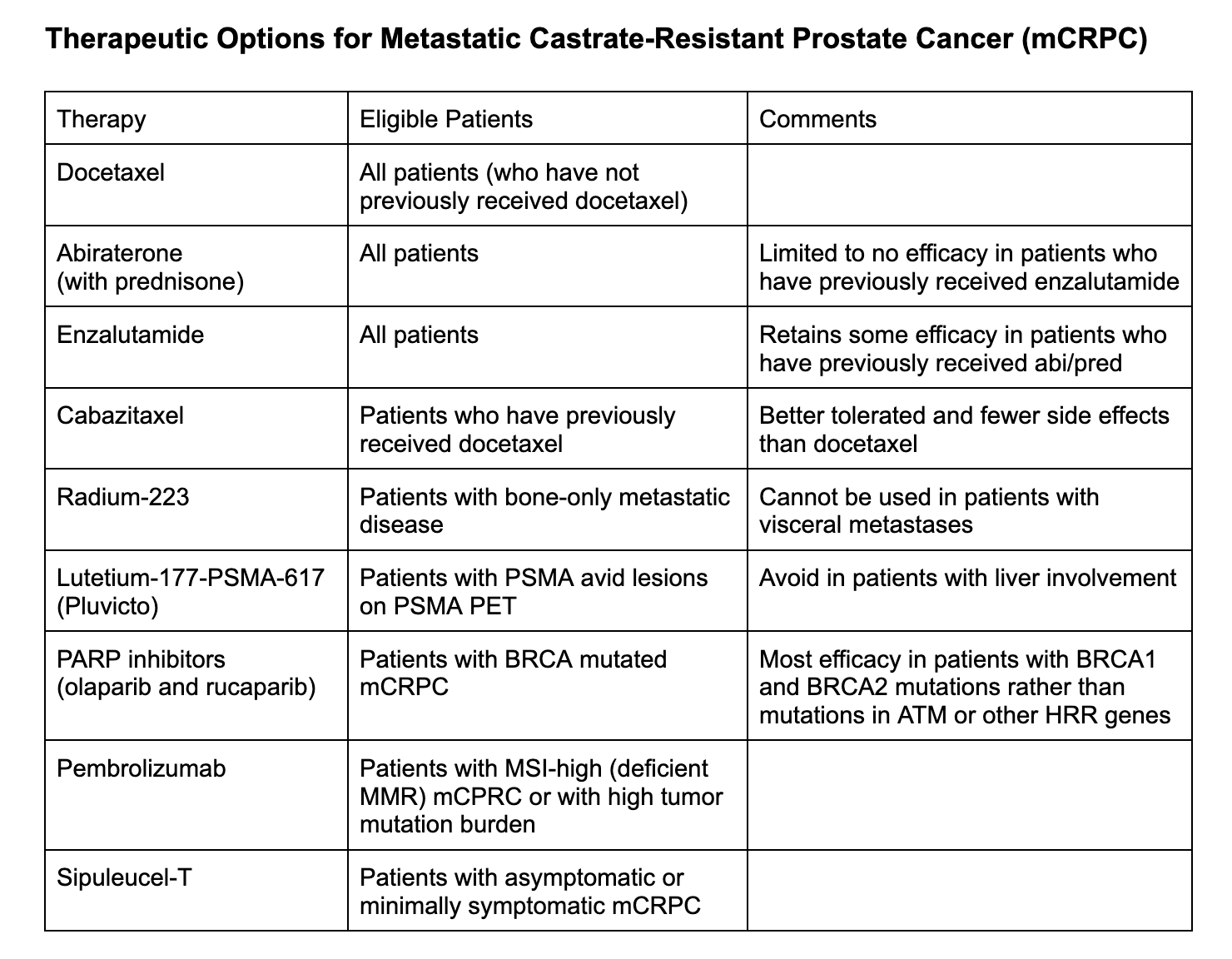
What is one treatment option for metastatic castrate-resistant prostate cancer in patients who did not receive docetaxel up front?
If the patient did not receive docetaxel up front, docetaxel will be a very reasonable option in the second-line setting
TAX 327 trial compared docetaxel vs. mitoxantrone chemotherapy in patients with castrate-resistant prostate cancer
Patients had improved overall survival with median 19 months compared to 16 months
What are the treatment options for metastatic castrate-resistant prostate cancer in patients who have already received docetaxel?
Can choose either enzalutamide or abiraterone + prednisone (abi/pred)
What is the evidence for abi/pred in the castrate-resistant setting?
Abi/pred was studied in two pivotal phase III trials demonstrating the efficacy of abi/pred + androgen deprivation therapy (ADT) compared to prednisone + ADT
Why was prednisone the comparator arm? Limited available treatment options at the time.
One trial evaluated patients with prior docetaxel
Median overall survival was 15 months with abi/pred + ADT compared to 11 months for prednisone + ADT
The other trial (COU-AA-302) had the same comparator arms but did not mandate prior docetaxel
Why wasn't the control arm investigator's choice? Prednisone + ADT seems like an inappropriate control arm.
There was improvement in overall survival and progression-free survival
Another endpoint was time to docetaxel, which was delayed in the abi/pred arm - this seems obvious when comparing an active combination against steroid monotherapy
When patients progressed, both groups got docetaxel
The issue is that time to docetaxel was delayed in the control arm. Patients had their disease progress on prednisone monotherapy when they could have been on active treatment with docetaxel
For the counter-argument that patients might not have been candidates for docetaxel - this is an argument for investigator’s choice, not withholding docetaxel from the entire arm
As a result, this trial did not answer the question of whether abi/pred is superior to docetaxel in the castrate-resistant setting
What is the evidence for enzalutamide in the castrate-resistant setting?
Enzalutamide was studied in two pivotal phase III trials demonstrating the efficacy of enzalutamide
The first trial (AFFIRM) compared enzalutamide vs. placebo in patients who had progressed on docetaxel
Median OS 18 months vs. 13 months favoring enzalutamide
These numbers are very similar to abi/pred in patients who already received docetaxel
The second trial (PREVAIL) compared enzalutamide vs. placebo but, like the COU-AA-302 abi/pred trial, did not mandate prior chemotherapy
There was improved one year progression free survival at 65% compared to 12% in the placebo arm
There was a delay in time to docetaxel and all other secondary endpoints including time to first skeletal related event
This is the same issue as with the COU-AA-302 abi/pred trial, namely that patients were receiving nothing instead of an active treatment like docetaxel
Should we choose docetaxel or abi/pred or enzalutamide in patients with castrate-resistant disease who have not received docetaxel?
Unclear - there is no evidence to demonstrate that abi/pred or enzalutamide is superior to docetaxel in this setting
Does the sequence of therapy matter? Can enzalutamide be given after abi/pred or vice versa?
There was a phase 2 crossover trial conducted in Canada in which patients got either abi/pred or enzalutamide first, then crossed over to the other drug at progression
100 patients in each arm
The median time to second PSA progression favored enzalutamide after progression on abi/pred (19 months) compared to abi/pred after progression on enzalutamide (15 months)
33% of patients had a response to enzalutamide in the second line while only 4% of patients had a response to abi/pred in the second line
Conclusion: abi/pred first, followed by enzalutamide, is reasonable as enzalutamide had activity and improved time to second PSA progression
However, abi/pred after enzalutamide has virtually no response rates and should not be considered a good treatment option
Why is abi/pred often preferred over darolutamide for triple therapy with ADT and docetaxel?
As discussed above, for patients who receive abi/pred as part of triple therapy, enzalutamide can still be given afterwards
What is the role of cabazitaxel in patients with metastatic castrate-resistant prostate cancer who have previously received docetaxel and either abi/pred or enzalutamide?
A phase III RCT (CARD trial) evaluated this question
Enrolled patients who had been exposed to docetaxel and a novel androgen signaling oral medication (either enzalutamide or abiraterone)
Compared cabazitaxel vs. the second line androgen signaling medication (enzalutamide in patients who had received abi/pred and vice versa)
Cabazitaxel improved overall survival at median 14 months compared to 11 months in patients receiving the second line androgen signaling medication
Cabazitaxel was better tolerated with fewer side effects than docetaxel
What is the role of radium-223 therapy in patients with metastatic castrate-resistant prostate cancer?
Radium-223 is a bone-seeking, alpha emitting radionuclide therapy for patients with bone-only metastatic disease
Radium resembles calcium chemically so it can form complexes with minerals in areas of increased bone turnover
Approval was based on the phase III ALSYMPCA trial
Randomized patients to six cycles of radium injections every 4 weeks vs. placebo
Patients had to be deemed ineligible for docetaxel, refused docetaxel chemotherapy, or previously received docetaxel
This trial was concurrently run with the trials of enzalutamide and abi/pred (hence why placebo was the comparator)
There was improvement in median overall by 3 months (14 months in radium arm vs. 11 months in placebo arm)
There was also delayed time to first symptomatic skeletal related event by 6 months
Conclusion: Radium therapy does do better than nothing in patients with bone-only metastatic disease
What is the role of lutetium-177-PSMA-617 therapy (Pluvicto) in patients with metastatic castrate-resistant prostate cancer?
Lutetium-177-PSMA-617 is a radioligand therapy that delivers beta-particle radiation to PSMA-expressing cells
As such, it can only be utilized in patients who have PSMA avid lesions on PSMA PET scan, including visceral metastases but not liver involvement
Approval was based on the phase III VISION trial
Patients were randomized to lutetium therapy vs. a restricted choice of options (enzalutamide, abi/pred, prednisone monotherapy)
Notably, chemotherapy was not an option; cabazitaxel is a good option in these patients and not all patients had previously received docetaxel
As a result, control arm appears to have been suboptimal
Many patients had progressed on two lines of oral therapies (abi/pred then enzalutamide) and were receiving one of them again, or had received enzalutamide and were put on abi/pred, which we already know has very limited efficacy
Additionally, there was a 55% withdrawal rate in the control arm, presumably because the control arm was suboptimal.
There was improvement in overall survival and progression free survival, with the above caveats
Conclusion: Lutetium is an effective therapy. Is it better than chemotherapy? We still do not know.
Additionally, lutetium is an expensive treatment and availability can be quite difficult
What is the role of poly ADP ribose polymerase (PARP) inhibitors in patients with metastatic castrate-resistant prostate cancer?
PARP inhibitors are utilized in patients with BRCA mutations
One important phase III trial evaluating PARP inhibitors in this setting was the PROfound trial
Randomized patients to olaparib vs. investigator’s choice of enzalutamide or abi/pred
Another phase III trial evaluating PARP inhibitors was the TRITON3 trial
Randomized patients to rucaparib vs. physician’s choice of docetaxel or abi/pred or enzalutamide
These trials included patients with BRCA1, BRCA2, and ATM mutations
However, subgroup analyses demonstrate that the majority of benefit occurred in patients with BRCA1 or BRCA2 mutations, rather than ATM mutation or other homologous recombination deficiency genes
Both trials demonstrated a progression free survival benefit for PARP inhibitors
Many of these patients did get chemotherapy after the trial was over, and once again some patients got abi/pred after enzalutamide
Patients in the control arm of these trials received the PARP inhibitor after progression
However, this delayed the initiation of something like docetaxel, which is known to improve overall survival
In the TRITON3 trial, there was no difference in overall survival!
In other words, patients were given a PARP inhibitor instead of potentially receiving a treatment that could have improved overall survival
Conclusions: PARP inhibitors do have activity, but ideally should be utilized after agents (e.g. docetaxel) that have demonstrated a survival benefit
What are the side effects of PARP inhibitors?
A third of patients on PARP inhibitors had grade 3 or higher anemia (hemoglobin < 8 g/dL)
Significant financial toxicity
Chart prepared by Neil Biswas, MD
References:
https://academic.oup.com/jnci/article/96/11/879/2520782 : Study looking at zoledronic acid in metastatic castrate-resistant prostate cancer
https://ascopubs.org/doi/10.1200/JCO.2013.51.6500 : Study looking at zoledronic acid in metastatic castrate-sensitive prostate cancer
https://jamanetwork.com/journals/jama/fullarticle/2595526: Zoledronic acid scheduling in metastatic prostate cancer
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(10)62344-6: Study comparing zoledronic acid and denosumab
https://www.nejm.org/doi/full/10.1056/nejmoa040720: TAX327 study that compared docetaxel vs. mitoxantrone chemotherapy in patients with castrate-resistant prostate cancer
https://www.nejm.org/doi/full/10.1056/nejmoa1014618 : Study evaluating abi/pred after docetaxel
https://www.nejm.org/doi/full/10.1056/nejmoa1209096 : Study evaluating abi/pred in metastatic setting (did not need docetaxel prior)
https://www.nejm.org/doi/full/10.1056/nejmoa1207506 : AFFIRM Trial evaluating enzalutamide
https://www.nejm.org/doi/full/10.1056/nejmoa1405095: PREVAIL Study evaluating enza (but did not require prior docetaxel)
https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(19)30688-6 : Canadian study that showed enza after abi/pred improved second PSA progression
https://www.nejm.org/doi/full/10.1056/nejmoa1911206 : Phase III CARD Study investigating the role of cabazitaxel in patients with met. castrate-resistant prostate cancer after prior therapy
https://www.nejm.org/doi/full/10.1056/nejmoa1213755 : Phase III ALSYMPCA study investigating radium-223 therapy
https://www.nejm.org/doi/full/10.1056/nejmoa2107322 : Phase III study that lead to approval of lutetium-177-PSMA therapy
https://www.nejm.org/doi/full/10.1056/NEJMoa1911440 : PROfound study for olaparib in BRCA-mutated met. castrate resistant prostate cancer
https://www.nejm.org/doi/full/10.1056/NEJMoa2214676 : TRITON study for rucaparib in BRCA-mutated met. castrate resistant prostate cancer
The crew behind the magic:
Show outline: Vivek Patel
Production and hosts: Ronak Mistry, Vivek Patel, Dan Hausrath
Editing: Resonate Recordings
Shownotes: Maria Khan, Neil Biswas, Ronak Mistry
Social media management: Ronak Mistry
We are proud to partner with HemOnc.org!
Want to learn more about the trials that lead to the regimens discussed today? What about dosing schedules? See links in the show notes for a link to HemOnc.org