Episode 042: Myeloma Series, Pt.3 - Intro to Multiple Myeloma
In this continuation of our myeloma series, we discuss the progression of MGUS & smoldering myeloma to multiple myeloma. We also outline how to risk stratify a patient with multiple myeloma and gauge their response to treatment.
This episode has been sponsored by Primum. To sign up for a free account, check out: tfoc.primum.co.
We highly recommend you check out our hemepath series to better understand some of this discussion!
What is the natural history of progression from MGUS to smoldering myeloma to multiple myeloma?
MGUS
Common! Occurs in ~5% of the adult population
Risk of progression of ~1% per year
Higher chance of progression if:
Non-IgG
M-spike > 1.5 g/dL
Abnormal FLC ratio
Smoldering Myeloma
Less common. Estimated to occur in 0.5% of adults over 40yo (Thorsteinsdottir et al., Blood 2021)
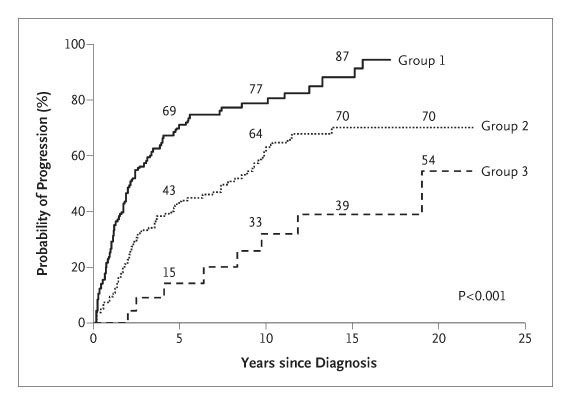
Risk of progression to multiple myeloma is highest in the first 5 years and decreases significantly after 10 years
Visual representation of the probability of MGUS and smoldering myeloma progression to multiple myeloma (Image from Figure 3 from Kyle et. al, NEJM, 2007; no copyright infringement intended)
How do we determine the number of plasma cells in a bone marrow biopsy?
Aspirate smears and flow cytometry will often underestimate the plasma cell percentage as plasma cells die during the aspiration process. However, flow cytometry is still useful for identification of abnormal plasma cell populations.
For the percentage of plasma cells, we rely on IHC and use a CD138 stain on the core biopsy
How do we risk stratify patients with multiple myeloma?
We check (in serum):
LDH
Albumin
Beta 2 microglobulin
Arguably more important, however, is FISH testing. The important chromosomal changes that portend a worse prognosis are:
t(14;16) and t(4;14)
Deletion 17p → remember that del 17p is associated with tp53 mutation
Amplification 1q (> 3 copies)
This data is then incorporated into the Revised International Staging System (R-ISS; ):
Revised International Staging System ([R-ISS]; Image from ASH Clinical News; no copyright infringement intended)
When risk stratifying a patient with MM, why do we need both FISH and karyotype testing?
The FISH testing gives us information about high-risk chromosomal changes important for prognosis and treatment.
Even though we don’t currently use the karyotype in our stratification systems, a complex karyotype may signify higher risk disease.
What are the levels of response to MM treatment?
Response criteria in multiple myeloma, as defined by the International Myeloma Working Group (adapted from Kyle and Rajkumar, Leukemia, 2009; no copyright infringement intended)
How do we define disease progression?
A >25% increase in:
Serum M spike
FLC ratio
24-hour UPEP
Bone marrow plasma cell percentage.
New plasmacytoma or bone lesions
Note that asymptomatic lab value elevations are defined as a progression event
How do we define the different responses to MM treatment?
PR: 50% reduction in the M spike
VGPR: you can detect an M spike but cannot quantify it or 90% reduction in the M spike, important goal for stem cell transplant
CR: requires a bone marrow biopsy, <5% clonal plasma cells
sCR: no clonal plasma cells in the bone marrow and no serum laboratory abnormalities
General approach to treatment (more to come in future episodes!):
Treat patients with “induction” regimen for 4-6 cycles
Goal is to achieve a VGPR before autologous transplant
Perform a bone marrow biopsy to determine if we have achieved CR
Goal: BM Plasma cells <10% before transplant
Why is testing for minimal residual disease important?
There is some thought that MRD negative patients may be able to back off on maintenance treatment. Whether MRD negativity is a surrogate for improved overall survival, however, remains a very open question.
How do we detect minimal residual disease?
The MRD testing techniques are NGF (Next Generation Flow) and NGS (Next Generation Sequencing).
NGF utilizes baseline immunophenotype information to get a sensitivity of ~1 in a million cells.
NGS can identify 1 in a million cells, and emerging data suggests that it may be able to get a sensitivity of 1 in 10 million cells. Unfortunately, at this time, it is very expensive and not widely available.
Note that multiple myeloma is not curable. But we hope to use risk stratification and MRD testing to keep patients from receiving unnecessary treatment.
References:
https://www.nejm.org/doi/full/10.1056/nejmoa01133202: 2002 article from Kyle et. al. discussing the risk of MGUS progression to multiple myeloma
https://www.nejm.org/doi/10.1056/NEJMoa070389?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200www.ncbi.nlm.nih.gov: 2007 article from Kyle et. al. discussing the risk of smoldering myeloma progression to multiple myeloma
https://ashpublications.org/blood/article/138/Supplement%201/151/478045/Prevalence-of-Smoldering-Multiple-Myeloma-Results: 2021 study from Thorsteinsdottir et. al where > 75,000 healthy patients were screened with SPEP and serum FLCs to determine that the prevalence of smoldering myeloma is ~0.5% in Icelandic adults > 40
https://www.nature.com/articles/bcj201592: 2015 article from Rajan and Rajakumar discussing the cytogenetic changes involved in MM development
https://ascopubs.org/doi/10.1200/JCO.2015.61.2267?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
https://ascopubs.org/doi/abs/10.1200/JCO.2022.40.16_suppl.8063: 2022 ASCO abstract from Emory suggesting that a complex karyotype portends a poor progression free survival and overall survival similar to high-risk FISH
https://pubmed.ncbi.nlm.nih.gov/18971951/z: 2009 article from Kyle and Rajkumar regarding response criteria in multiple myeloma
https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(21)00535-0/fulltext: FORTE trial; more to come on treatment in a later episode!
https://biomarkerres.biomedcentral.com/articles/10.1186/s40364-021-00328-2/tables/1: Table outlining the pros and cons of different MRD testing modalities
https://ashpublications.org/blood/article/140/Supplement%201/2108/489958/Prospective-Trial-Using-Multimodal-Measurable: 2022 ASH abstract discussing using NGS to guide MM maintenance therapy discontinuation
https://ashpublications.org/blood/article/140/Supplement%201/7304/492868/Free-from-Maintenance-Drug-Therapy-in-Multiple: 2022 ASH abstract discussing using NGS to guide MM maintenance therapy discontinuation
https://ashpublications.org/blood/article/140/Supplement%201/2397/487888/Maintenance-Therapy-Cessation-for-Sustained-MRD: 2022 ASH 2022 Abstract uses NGF to guide MM maintenance therapy discontinuation
The crew behind the magic:
Show outline: Vivek Patel
Production and hosts: Ronak Mistry, Vivek Patel, Dan Hausrath
Editing: Vivek Patel
Shownotes: Madeline Fitzpatrick, Ronak Mistry
Graphics, social media management: Ronak Mistry
This episode has been sponsored by Primum. To sign up for a free account, check out: tfoc.primum.co.